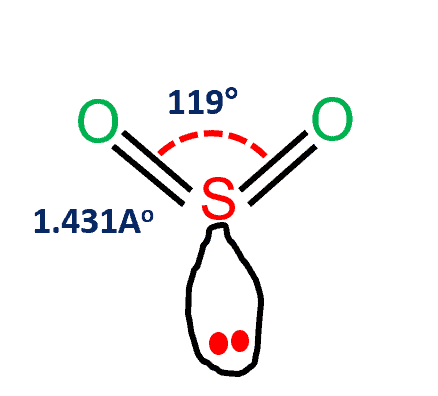
Sulfur dioxide (SO2) is a covalent molecule. Sulfur and oxygen atoms with identical electronegativity try to link together. As a result of the small variation in electronegativity between the two atoms, electron bonds are shared, producing covalent bonds.
| Name of molecule | Sulfur dioxide (SO2) |
| Bond Angles | 119 degrees |
| Molecular Geometry of SO2 | Trigonal planar |
| Hybridization of SO2 | sp2 hybridization |
| No Valence Electrons in the molecule | 18 |
| SO2 Polar or Nonpolar | Polar |
| The dipole moment of SO2 | 1.61 debye |
| SO2 Ionic or Covalent? | covalent |
Table of Contents
The polarity of the SO2 molecule
In SO2 molecules, the electronegative potential of oxygen is higher than that of sulfur.
As a result, oxygen exerts a stronger pull on sulfur dioxide’s covalent bonds.
The side of the molecule containing both oxygen atoms is somewhat negatively charged.
whereas the portion that has the sulfur atom has a slightly positive charge.
This makes SO2 a polar covalent molecule.
In addition, the unbonded electrons on the sulfur and oxygen create repulsion between atoms.
This is another cause of the polarity of the sulfur dioxide molecule.
SO2 Ionic or Covalent?
SO2 is a covalently bonded molecule.
To determine whether a bond is covalent or ionic, we must understand the difference in electronegativity. A covalent bond is formed when the electronegativity difference between two elements ranges between 0 (two of the same element bonding together) and 1.69 (almost ionic bonding).
As a side note, the greater the electronegativity difference, the more polar the bond will be. Since oxygen has an electronegativity of 3.5 and sulfur has an electronegativity of 2.6, the electronegativity difference between the two elements is 0.9. As a result, SO2 is a covalent compound and slightly polar as well.

Electronegativity versus Bond Type
The absolute value of the difference in electronegativity (EN) of two bound atoms gives an approximate estimate of the polarity of the bond and, consequently, the bond type.
The bond is covalent and nonpolar when the difference is extremely tiny or nil. The bond is polar covalent or ionic when it is big.
Examples
- H-H bond, EN is zero, so the bond is nonpolar.
- H-Cl bond, EN is 0.9, so the bond is polar covalent.
- Na-Cl bond, EN is 2.1, so the bond is ionic.
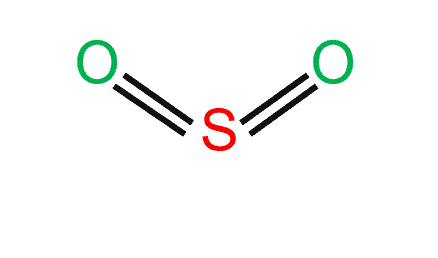
SO2 Molecular Geometry
SO2 has a bent molecular shape, with a bond angle of 120°. The distribution of charges on the central atom is asymmetrical. The portion of the molecule that contains both oxygen atoms is somewhat negatively charged. The sulfur atom, on the other hand, has a slightly positive charge. On sulfur, there is also a single pair of electrons.
Between sulfur and each oxygen atom, one sigma and one pi bond are formed between sulfur and each oxygen atom. To reduce repulsions, the double bonds and the lone pair are separated as much as possible, and so the molecule is bent.
What is Sulfur dioxide (SO2)
Sulfur Dioxide (American English), also known as Sulfur Dioxide (Commonwealth English), is a bond between sulfur and oxygen atoms. SO2 is a colorless, toxic inorganic gas with a terrible odor that is comparable to that of nitric acid. This gas is naturally released by volcanic activity.
When dissolved in water, it produces a weak acid solution.
Sulfur dioxide is a key precursor of sulfuric acid and is found at modest levels in the environment.
The molar mass of Sulfur dioxide
The molar mass of S =32.066 g/mol.
O2 molar mass = 16.00 x 2 = 32.00 g/mol.
Molar mass of SO2 = 64.066 g/mol
Key Points
- SO2 Lewis structure would comprise two atoms of oxygen (O) and one sulfur atom. The number of valence electrons in both S and O atoms is six. The total number of SO2 valence electrons is 12.
- The electron geometry of SO2 is formed in the shape of a trigonal planner.
- The three pairs of bonding electrons are arranged in the plane at an angle of 120 degrees.
- The sulfur’s valence electron is = 6.
- valence electrons of oxygen = 6 (There are 2 oxygen atoms in the compound.)
Production of sulfur dioxide
Sulfur or iron pyrites are burnt in the air to produce SO2.
S + O2 (gas) —> SO2 (gas)
4FeS2 + 11O2 –> 2Fe2O3 (solid) + 8SO2 (gas)
Similarities between Sulfur and Oxygen atoms
- Both O and S have the same outer electric configuration of ns2 and np4.
- O and S are usually divalent.
- Both exhibit an allopatric form.
- O and S are non-metals.
- In reaction with metals, they both react with the oxidation state of-2.
- While reacting with nonmetals, both form covalent compounds, for instance, H2O, H2S, CO2, and CS2.
Dissimilarities between oxygen and sulfur
| Oxygen | Sulfur |
| Two allotropic forms | 3 allotropic forms |
| Gas at ordinary temperature | Solid at ordinary temperature |
| Sparingly soluble in water | Not soluble in water |
| Helps in combustion | Combustible itself |
| Paramagnetic in nature | diamagnetic in nature |
| Does not react with water | When steam is passed through boiling sulfur, a little hydrogen sulfide and sulfur dioxide is formed. |
| Does not react with acids | It is readily oxidized by concentrated sulfuric acid or nitric acid. |
Sulfur dioxide Effects on Humans
Sulfur dioxide is a poisonous gas that is hazardous to human health.
It can irritate the eyes, nose, throat, and lungs’ skin and mucous membranes.
Its excessive amounts might irritate and induce inflammation in the respiratory system.
Sulfur dioxide emissions in the air can lead to the creation of other sulfur oxides (SOx).
SOx can react with other compounds in the atmosphere to form small particles.
These small particles may penetrate deeply into the lungs, and their sufficient quantity can contribute to health problems.
Summary
- Sulfur dioxide (SO2) is a covalent molecule.
- The molecular geometry of sulfur dioxide is a bent shape.
- The sulfur dioxide molecule has two double bonds between the sulfur atom and oxygen atoms.
- There are five lone pairs of electrons in the molecule of SO2.
- SO2 gives a weak acid solution when dissolved in water.
More Interesting Topics
Molar Mass of Acetic Acid| Easy-Explanation
Concentration Gradient Definition
Sodium Phosphate – Formula, Structure, Types, and Uses
Sulfurous Acid| Formula & Lewis Structure
Valence Electrons in Nitrogen
Frequently Asked Questions (FAQs)
Some of the frequently asked questions are given below. If you have any questions, feel free to comment or send an email to [email protected]
1. How many lone pairs of electrons are present in the SO2 Lewis structure?
There are two double bonds between the sulfur and oxygen atoms in the sulfur dioxide molecular geometry. Each Sulfur atom has one lone pair, while each Oxygen atom has two. (Read full article SO2 lewis structure)
2. What is the biggest source of SO2?
Burning fossil fuels are the largest source of SO2 in the atmosphere.
3. Is MgCl2 ionic or covalent?
When the magnesium atom loses two electrons to create the Mg2+ ion and each chlorine receives one electron to form the Cl– ion, an ionic bond is formed between the magnesium and chlorine atoms.
Check “Is MgCl2 ionic or covalent?”.
4. Is BF3 polar or nonpolar?
BF3 is a non-polar compound. In BF3, the central boron atom has sp2 hybridized orbitals, resulting in an unfilled p orbital on the Bron atom and trigonal planar molecular geometry. Because the Boron-Fluorine bonds are all 120 degrees apart, any net dipole in that plane is canceled out. Even if each B-F bond is polar, the net dipole moment is zero because adding the bond vectors cancels everything out.
Check the full article here“Is BF3 polar or nonpolar?”.
5. What is Sulfur hexafluoride?
Sulfur hexafluoride (SF6) is a non-toxic gas that is used in a variety of applications due to its inert properties. While SF6 is non-toxic when used properly, toxic byproducts can be produced during electrical discharges within SF6-filled equipment, posing a threat to the health of workers who come into contact with them.
6. What is Sulfur trioxide?
SO3 (sulfur trioxide) is a chemical compound. It is available in three forms: gaseous monomer, crystalline trimer, and solid polymer. It is solid at just below room temperature and has a relatively narrow liquid range. Gaseous SO3 is the primary precursor to acid rain.
7. NH3 is a polar or nonpolar molecule?
Ammonia (NH3) is a polar molecule. Since the molecule contains three N-H bond dipoles that do not cancel out. In each bond, nitrogen is more electronegative than hydrogen. This unequal distribution of charges among both nitrogen and hydrogen atoms makes NH3 a polar molecule.
8. CH4 polar or nonpolar?
Methane (CH4) is a nonpolar molecule. Nonpolar molecules form when electrons in a diatomic molecule are shared equally or when polar bonds in a bigger molecule cancel each other out.
9. Is SiCl4 polar or nonpolar?
SiCl4 (silicon tetrachloride) is a nonpolar molecule. Because the four chemical bonds between silicon and chlorine are uniformly distributed, SiCl4 is non-polar. A polar covalent bond is a type of covalent link that is intermediate between pure covalent bonds and ionic bonds. When the difference in electronegativity between the anion and the cation is between 0.4 and 1.7, such bonds occur.
Check the full article here“Is SiCl4 polar or nonpolar?”.
10. What is nitrogen trifluoride gas?
Nitrogen trifluoride (NF3) is an odorless, colorless gas. When inhaled, it is exceedingly poisonous. Containers may burst violently if exposed to fire or heat for a lengthy period of time.
Because sulfur and oxygen are both non-metals, the formation of sulfur dioxide is a covalent bonding process. Two oxygen atoms encircle the sulfur atom in the middle.
Related Links
SiO2 Lewis Structure
N2O Lewis Structure| Laughing Gas
HCN Lewis Structure
Ionic Compounds| Properties, and Examples
What is the molar mass for sulfur dioxide?
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023