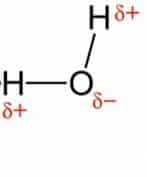
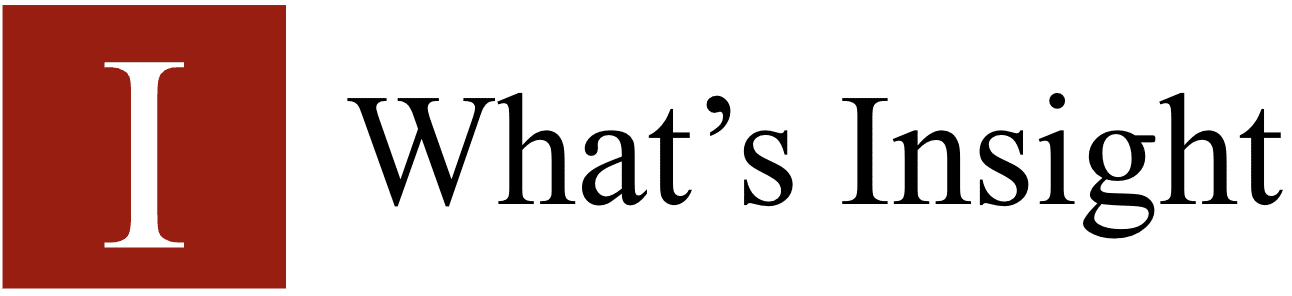Sigma bonds (bonds) are the strongest covalent chemical bonds in chemistry. They are created by atomic orbitals colliding head-on. The sigma bond symbol is σ
Due to the direct overlapping of the involved orbitals, sigma bonds are the strongest covalent bonds. The electrons that participate in a bond are referred to as electrons.
All single bonds are, in general, sigma bonds. The following are the characteristics of the sigma bond:
- A sigma bond is a covalent bond produced by collinear or coaxial overlapping of an atomic orbital in a line of the internuclear axis.
- Due to the direct overlapping of the involved orbitals, sigma bonds are the strongest covalent bonds.
- The electrons that participate in a σ bond are referred to as σ electrons.
Table of Contents
Sigma Bond vs Pi bond
The sigma bond is a covalent link produced by the head-on overlapping atomic orbitals. The pi bond is a covalent connection created by the lateral overlapping of half-filled atomic orbitals of atoms.
The lateral overlap of the atomic orbitals that are aligned perpendicular to the internuclear axis forms a pi bond, thus the extent of orbital overlapping in a sideways manner is smaller.
As a result, the sigma ( σ ) bond is more powerful than the Pi (π) bond.
CO2 Sigma and Pi Bonds
There are two double bonds in carbon dioxide. One sigma bond and one Pi bond combine to form a double bond.
Types Of Overlapping
- S-S overlapping (One ‘s’ orbital from each participating atom experiences head-on overlapping along the internuclear axis in this type of overlapping. Before one s orbital may overlap with another, it must be half-filled. “
- S-P overlapping (Along the internuclear axis, one half-filled s orbital overlaps with one half-filled p orbital, producing a covalent bond.)
- P-P overlapping (Along the internuclear axis, one half-filled p orbital from each participating atom experiences head-on overlapping.)
Similarities between sigma bonds and pi bonds
Sigma bonds and pi bonds are both based on certain molecular orbitals that are produced from the overlapping of specific atomic orbitals, such as s orbitals for sigma bonds and p orbitals for pi bonds. They can also be stable or unstable depending on whether electrons are in bonding or anti-bonding molecular orbitals.
Differences between sigma bonds and pi bonds
| Sigma Bond | Pi Bond |
| Atomic orbitals overlap along the bonding axis | Atomic orbitals overlap above and below the bonding axis |
| The first bonds that form inside molecules are between atoms. | Second bonds form between atoms within molecules |
| Formed from overlapping orbitals such as s orbitals | Formed from overlapping orbitals such as p orbitals |
| Overlapping orbitals perpendicular to pi bond orbitals | Overlapping orbitals perpendicular to sigma bond orbitals |
Frequently Asked Questions
Some of the frequently asked questions are given below:
1. How many sigma bonds are in a triple bond?
One sigma bond and two pi bonds make up a triple bond.
2 How to count sigma and pi bonds?
The following rules are all you need to know:
A single bond is equal to one sigma bond.
A double bond is made up of one sigma and one pi bond.
1 sigma and 2 pi bonds equal a triple bond.
3. What is a sigma bond example?
The triple bond between the two nitrogen atoms in the molecule nitrogen (N2), for example, consists of a sigma bond and two pi bonds.
4. What is the difference between a sigma bond and a pi bond?
- Electrons in sigma bonds are distributed along the axis that connects the connected nuclei, whereas electrons in pi bonds are distributed above and below the axis but not along it.
- Sigma bonds are the first to form between atoms within molecules, followed by pi bonds.
- Sigma bonds are frequently formed by combining “s” orbitals from distinct atoms, whereas pi bonds are formed by combining p and similar orbitals from different atoms.
5. Electric field units?
In the meter-kilogram-second and SI systems, electric field units are newtons per coulomb, which are equivalent to volts per meter.
6. Paramagnetic materials?
Paramagnetic materials are metals that are weakly attracted to magnets. Aluminum, gold, and copper are among them.
7. Is chlorine a metal?
Chlorine is nonmetal since it lacks metal-like properties such as electrical conductivity, flexibility, and strength.
8. Calcium electron configuration?
Calcium is a silvery-white, soft metal that tarnishes rapidly in the air and reacts with water.
calcium electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2
9. How many electrons does helium have?
In a single Helium atom, there are 2 protons, 2 electrons, and 2 neutrons. Helium is the second element of the periodic table. Check the full article “How many electrons does helium have?”.
10. Valence electrons in nitrogen?
Nitrogen has 5 valence electrons. Nitrogen (N) is a nonmetallic element in Periodic Group 15 [Va]. It is a colorless, odorless, and tasteless gas that is the most abundant element in the Earth’s atmosphere and is found in all living things.
Related Topics
Co2 Polar Or Nonpolar
SO2 Polar Or Nonpolar
CH4 Lewis Structure & Molecular Geometry
SO2 Lewis Structure| 4 Simple Steps
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023