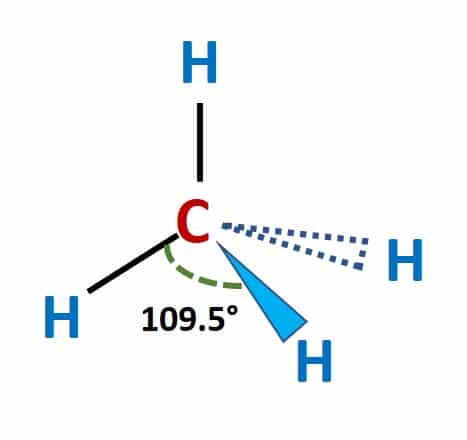
Methane (CH4) is a colorless, odorless, and highly combustible gas that is utilized to generate energy and heat houses all over the world. The CH4 Lewis structure comprises two different atoms: carbon and hydrogen. It is a nonpolar molecule with a bond angle of 109.5°. CH4 is utilized in chemical processes to create other essential gases such as hydrogen and carbon monoxide, as well as carbon black, a chemical component present in some types of rubber used in automobile tires.
| Name of molecule | Methane (CH4) |
| Bond Angles | 109.5° degrees |
| CH4 Molecular geometry | Tetrahedral |
| Hybridization of Methane | sp3 hybridization |
| Number of Valence Electrons in the molecule | 8 |
Table of Contents
Step by Step Construction of Lewis Structure
The following are the steps to constructing the Lewis Structure.
Step-1: Count the valence electrons of atoms
To draw the Lewis structure, we need to figure out the number of valence electrons in individual atoms as shown in the table below.
| Atom | Electronic Configuration | Valence Electrons (VEs) |
| 6C | 1S2 2S1 2P3 | 4 |
| 1H | 1S1 | 1 |
VEs = VEs in hydrogen + VEs in carbon + VEs in hydrogen atoms
Valence electrons = 4+4(1) = 8 electrons.
Step-2: Determine the central atom
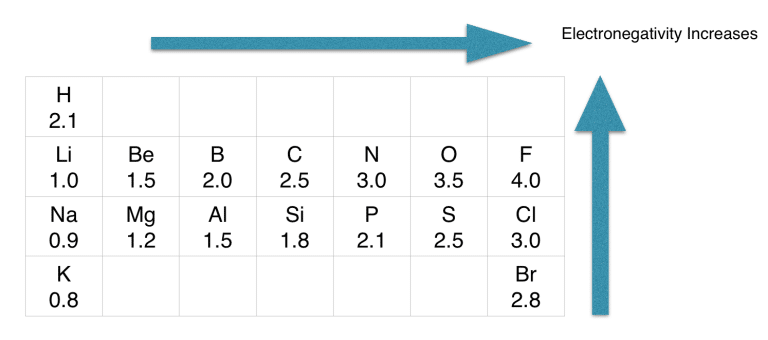
If we check the proper arrangement of C and H in the periodic table, we will find that the electronegativity values of C and H are 2.5 and 2.1.
As per the rule, the atom with the least electronegative value should come at the center of the structure.
Since hydrogen is the least electronegative, it can not take a central position.
Therefore, carbon will take the central position.
Step-3: Place electron pairs between the atoms
We need to distribute the 8 remaining valence electrons. Each hydrogen atom will have one electron, and carbon will have four electrons.
Step-4: Place the remaining electrons around the other atoms.
After making a single bond with four hydrogen atoms, C is left with no valence electrons.
CH4 Molecular Geometry
Methane has a tetrahedral molecular geometry with bond angles of 109.5°. There are four covalent bonds between hydrogen atoms and central carbon atoms in the methane molecule. According to the Valence shell electron pair repulsion (VSEPR) theory, the same kind of electron pair repels each other. As there are four bonding pairs of electrons, they adopt the tetrahedral molecular shape to minimize their repulsive forces.
CH4 Lewis Structure- Key Points
- The colorless and odorless gas
- It is extremely combustible and is utilized to generate energy.
- also known as marsh gas
- Density = 0.657 kg/m³
- Boiling point -161.6 °C
- Freezing point: -182 °C
- Molar mass is 16.04 g/mol
- In the CH4 lewis structure, there is a single bond between carbon and hydrogen atoms
CH4 Hybridization
There are four sigma bonds between C and H. The carbon atom in the center is sp3 hybridized. This is because, in the valence shell of carbon, one 2s orbital and three 2p orbitals combine to produce four sp3 hybrid orbitals of equal energy and shape. In addition, four H atoms utilize these four carbon sp3 hybrid orbitals to create C-H sigma bonds, which leads to the creation of the methane molecule.
Molar Mass of CH4
Molar mass of H = 1.00794 g/mol
Carbon molar mass = 12.011 g/mol
The CH4 molar mass is 16.04 g/mol.
Is CH4 Polar or Nonpolar?
CH4 is a nonpolar molecule.
With four identical C-H bonds, CH4 forms a symmetric tetrahedral geometrical geometry. Carbon and hydrogen have electronegativity of 2.55 and 2.2, respectively, resulting in almost zero partial charges.
Methane Effects
Methane (CH4) poisoning can occur after prolonged exposure to high quantities of methane. While it is regarded to be generally non-toxic, its main danger is that it acts as an asphyxiant, comparable to carbon monoxide exposure.
CH4 Intermolecular Forces
CH4 intermolecular forces are London dispersion forces.
Generally, the most important intermolecular forces are dipole-dipole interactions and London dispersion forces. CH4 does not have dipole-dipole interaction since C and H have such similar electronegativities and C-H bonds are nonpolar.
Summary
To summarize everything in this article, the following are some important points:
- In the CH4 Lewis structure, the methane molecule has four single shared covalent connections between the carbon and hydrogen atoms.
- The bond angle is 109.5 degrees, and there are 8 valence electrons.
- CH4 is a nonpolar molecule with tetrahedral geometry.
- Exposure to methane can be dangerous.
Related Links
CO2 Lewis Structure and Molecular Geometry
SiO2 Lewis Structure
SO2 (Sulfur Dioxide) Lewis structure
N2O Lewis Structure| Laughing Gas
HCN Lewis Structure & Molecular Geometry
Frequently Asked Questions (FAQs)
Some of the frequently asked questions are given below:
1. Why Methane is nonpolar?
Natural gas’s primary component, methane, is a nonpolar molecule. In a three-dimensional configuration fashioned like a four-sided pyramid, four hydrogen atoms encircle a single carbon atom. The symmetry of the hydrogens on the pyramid’s corners distributes electric charge uniformly across the molecule, making it nonpolar.
2. Explain Methane Lewis Structure in simple words
The methane (CH4) molecule has four single shared covalent bonds between the carbon and hydrogen atoms. Furthermore, because there are no lone pairs of electrons on an atom, the bond angle is the ideal tetrahedral angle of 109.5°.
3. What is Methane?
Natural gas, which is largely made up of methane, is the most environmentally friendly fossil fuel. Methane that is discharged into the atmosphere before being burnt, on the other hand, is hazardous to the environment. Methane contributes to climate change because of its ability to trap heat in the atmosphere.
4. Why Lewis structures are important?
The Lewis structure is a simplified representation of valence shell electrons.
It depicts the arrangement of electrons around individual atoms in a molecule.
Electrons are shown as “dots” or as a line between two atoms when they are bonded.
5. How to draw Lewis’s structure of oxygen?
In the O2 Lewis structure, there is a double bond between two oxygen atoms.
Oxygen is a diatomic nonpolar molecule with a bond angle of 180 degrees.
In its molecule, both oxygen atoms have the same electronegativity value, and both atoms share equal ratios of bonded shared electrons, so the overall O2 molecule turns out to be nonpolar in nature.
6. What is the dot structure of Hydrogen Sulfide?
On both sides of the central sulfur atom in the H2S Lewis structure, there are two hydrogen atoms.
The molecule bends due to the existence of two unbonded pairs of electrons.
The molecule is slightly polar because sulfur is more electronegative than hydrogen.
In the case of H2S, the vectorial sum of the bond dipole moments results in a non-zero total dipole moment. As a result, dipole-dipole interactions are observed in hydrogen sulfide.
7. Is CH4 polar?
The CH4 molecule is symmetrical and lacks a loan pair, Therefore, CH4 is a nonpolar molecule.
8. What is CLF3 molecular geometry?
CLF3 has a T-shaped molecular geometry and trigonal bipyramidal electron geometry. According to the ClF3 Lewis structure, this molecule has two lone pairs and three bound pairs, according to the ClF3 Lewis structure. ClF3 is a polar compound.
Author
Umair Javed
Umair has been working at Whatsinsight since 2020 as a content writer.
He has a Master’s degree in Materials Science.
More on Lewis Structures
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023


