Nitrogen trifluoride (NF3) is a colorless gas that has a moldy odor. It is extremely toxic when inhaled. When exposed to fire or heat for an extended period of time, the containers may rupture violently and rocket. NF3 is used as a Fluorine source in the electronics industry and in high-power lasers.

| Name of molecule | Nitrogen trifluoride (NF3) |
| Bond Angles | 102 degrees |
| Molecular Geometry of NF3 | Tri angular planner (Due to lone pair-bond pair repulsion) |
| Hybridization of NF3 | SP3 |
| NF3 valence electrons | 8 valence electrons |
| NF3 dipole moment | 0.23 Deby |
Although NF3 is relatively stable at room temperature, its reactivity changes when heated. It is described as having similar reactivity to oxygen up to 200 °C, but above that temperature it tends to dissociate significantly into NF2 and F radicals, transforming it into a strong oxidizing agent.
NF3 does not react with water, dilute acid or alkali, glass, or mercury. At 350°C, it does not react with H2, but the mixture explodes when sparked. It is less toxic to breathe than nitrogen oxides (NOx), but it oxidizes hemoglobin to methemoglobin (which reduces oxygen-carrying in the blood). It does not, unlike ammonia, act as a ligand to transition metals and form complexes [Reference].
Table of Contents
More Interesting Topics
N2 Lewis Structure| Hybridization & Molecular Geometry
The Molar Mass of Nitrogen
Valence Electrons in Nitrogen
NH3 Molecular Geometry| Trigonal Pyramidal
Silicon Carbide – An Overview
Frequently Asked Questions
1. Why do NH3 and NF3 have different bond angles?
Hydrogen is less electronegative than nitrogen in NH3, whereas fluorine is more electronegative than nitrogen in NF3. As a result, in the NH3 molecule, nitrogen pulls electrons away from hydrogen, whereas fluorine pulls electrons away from nitrogen in the NF3 molecule. As a result, bond pair-bond pair repulsion is greater in NH3 than in NF3.
As a result, the bond angle in NF3 decreases from 107° in ammonia to 101.9° because the very electronegative fluorines pull the electrons in the N-F bonds towards themselves, reducing interelectronic repulsions. For details about NH3 molecular geometry, check the article NH3 Lewis structure.
2. Why NF3 is less polar than NH3?
The reason for this is fluorine’s high electronegativity. Because F is more electronegative than N, the bond moments (dipoles) are directed in the opposite direction to the lone-pair, resulting in NF3 having a dipole moment of 0.234 Debye, compared to ammonia’s dipole moment of 1.47 Debye. Because hydrogens in ammonia are less electronegative than nitrogen, the bond dipoles are polarised in the same way as the lone pair. Because of its reduced polarity, it is clearly a poorer (potential) ligand. Another way to look at it is that the highly electronegative fluorines reduce the electron density of nitrogen, making it less electron-rich.
3. Is Nh3 Polar?
NH3 is polar due to the presence of three non-zero N-H bond dipoles. Nitrogen is more electronegative than hydrogen in each bond. Polarity results from the unequal charge distribution of nitrogen and hydrogen atoms.
4. h2s molecular geometry?
The molecular geometry of H2S is bent because the two lone pair electrons on the sulphur central atom repel each other and the electrons of the next two bonded pair electrons. As a result, these electron pairs (lone pair and bond pair) take the position where the repulsion is the least and they become stable (no further repulsion force exists in electrons pairs).
5. Is SiCl4 polar or nonpolar?
SiCl4 (silicon tetrachloride) is a nonpolar molecule. Because the four chemical bonds between silicon and chlorine are uniformly distributed, SiCl4 is non-polar. A polar covalent bond is a type of covalent link that is intermediate between pure covalent bonds and ionic bonds. When the difference in electronegativity between the anion and the cation is between 0.4 and 1.7, such bonds occur.
Check the full article “Is SiCl4 polar or nonpolar?”.
6. What is the oxidation number of NH3?
Ammonia is made up of one nitrogen atom and three hydrogen atoms (NH3). The NH3 oxidation number is zero because the sum of the individual oxidation values of the atoms nitrogen (oxidation number = -3) and hydrogen (oxidation number = 1) is zero.
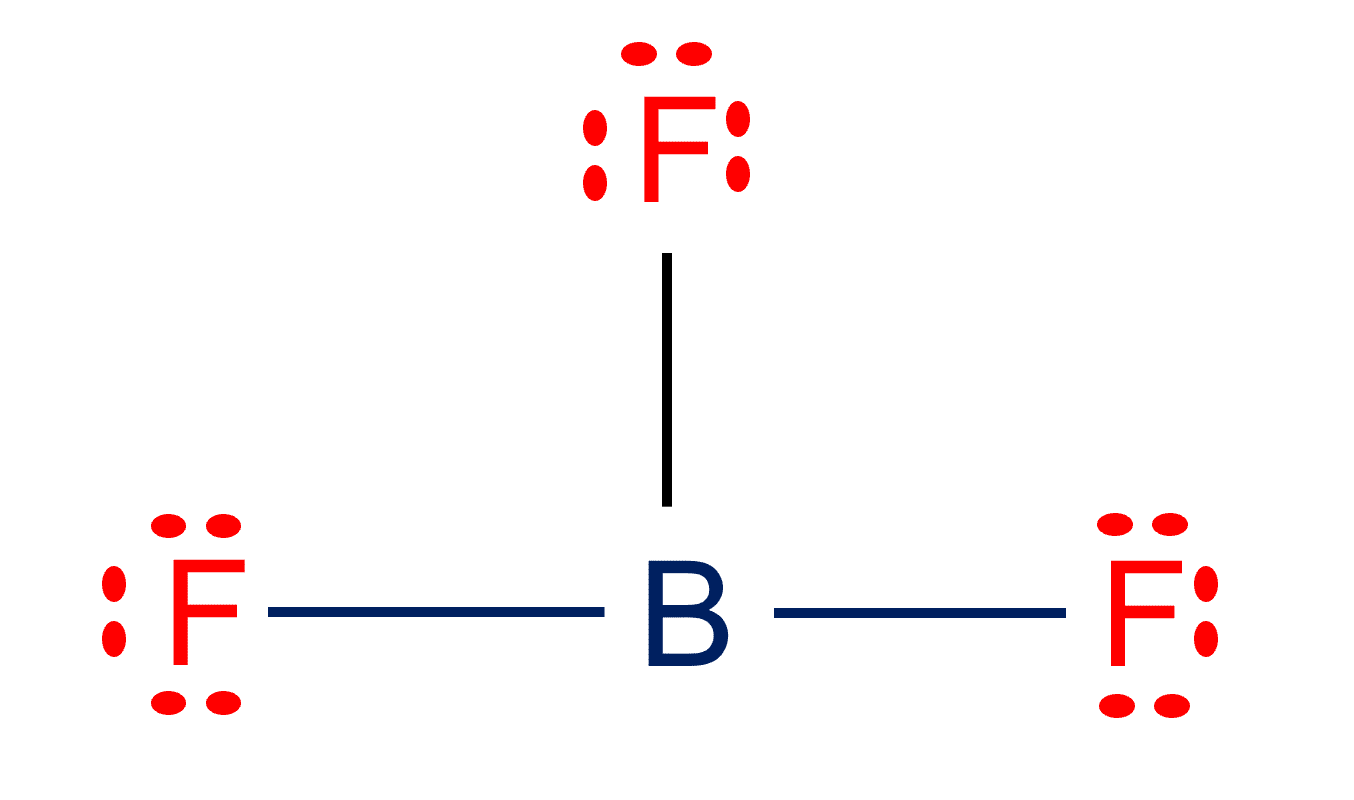
7. Is BF3 Polar or Nonpolar?
BF3 is a non-polar compound. In BF3, the central boron atom has sp2 hybridized orbitals, resulting in an unfilled p orbital on the Bron atom and trigonal planar molecular geometry. Because the Boron-Fluorine bonds are all 120 degrees apart, any net dipole in that plane is canceled out. Even if each B-F bond is polar, the net dipole moment is zero because adding the bond vectors cancels everything out.
Check the full article “Is BF3 polar or nonpolar?”.
8. How cold is liquid nitrogen?
Liquid nitrogen is nothing more than extremely cold nitrogen. The temperature is 320 degrees Fahrenheit below zero (-196oC). It’s so cold that anything it comes into contact with instantly freezes.
Check the full article “How cold is liquid nitrogen?”.
9. What is nitrogen trifluoride gas?
Nitrogen trifluoride (NF3) is an odorless, colorless gas. When inhaled, it is exceedingly poisonous. Containers may burst violently if exposed to fire or heat for a lengthy period of time.
10. What is perchloric acid used for?
Perchloric acid (HCLO4) is used to separate potassium from sodium in many laboratory tests and industrial processes. It is also used as an oxidizing agent, especially in the determination of chromium in steel, ferrochrome, chromite, and leather.
More Interesting Topics
Molar Mass of Acetic Acid| Easy-Explanation
Concentration Gradient Definition
Sodium Phosphate – Formula, Structure, Types, and Uses
Sulfurous Acid| Formula & Lewis Structure
Valence Electrons in Nitrogen
Charge of Ammonia (NH3)| Simple Steps
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023