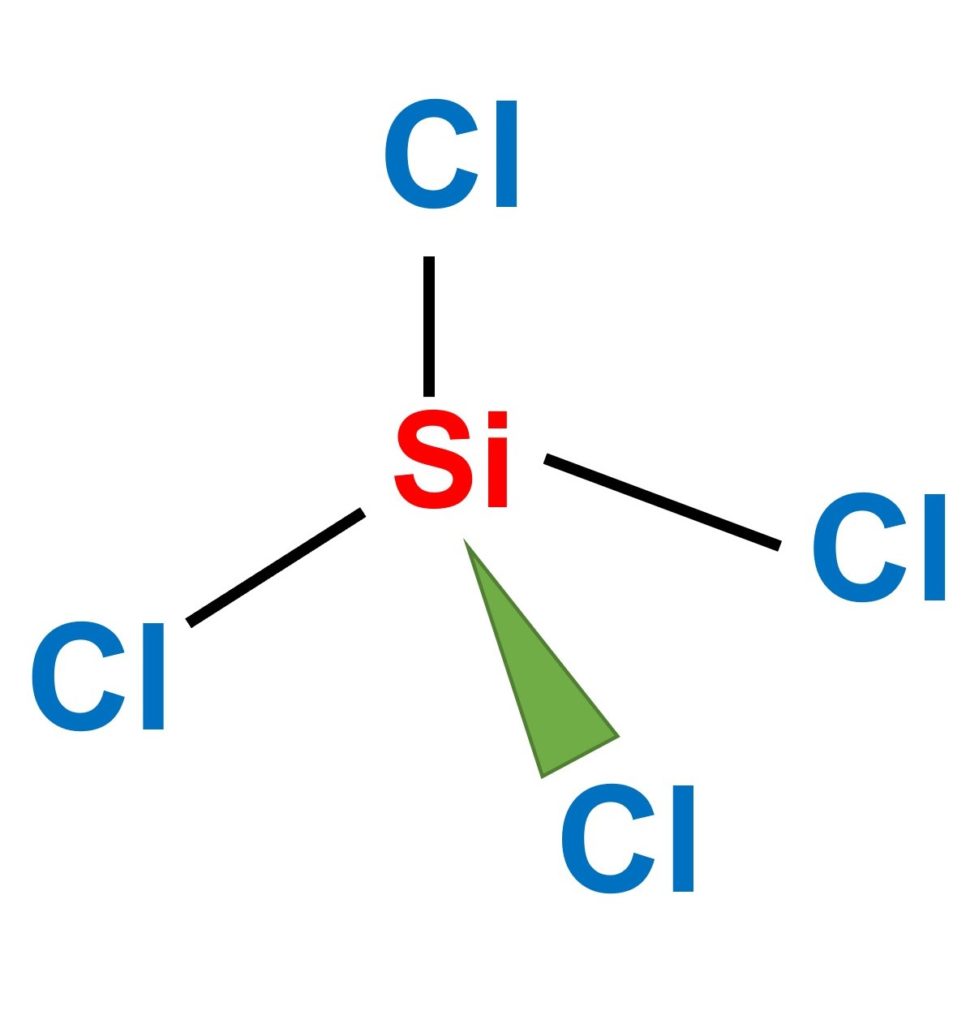
SiCl4 (Silicon tetrachloride) is a non-polar molecule. SiCl4 is non-polar because the four chemical bonds between silicon and chlorine are equally distributed. Keep reading to know more about the question “SiCl4 polar or nonpolar”.
At ambient temperature and normal air pressure, silicon tetrachloride is a liquid, although it is volatile enough to emit vapors.
It is not soluble in water and, when combined with water, produces silicon dioxide and hydrochloric acid. In benzene, chloroform, ether, and hydrochloric acid, silicon tetrachloride is soluble.
It’s most typically used to purify elemental silicon, which is then used in microchips and other computer applications.
| Name of molecule | SiCl4 (Silicon tetrachloride) |
| SiCl4 Bond Angles | 109° |
| Molecular Geometry of SiCl4 | tetrahedral |
| Hybridization of SiCl4 | Sp3 hybridization |
| No of Valence Electrons in the SiCl4 | 32 |
| The dipole moment of SiCl4 | zero |
Table of Contents
Frequently Asked Questions
1. What is Silicon carbide?
Silicon carbide (SiC), often known as carborundum, is a silicon-carbon semiconductor. Moissanite, an incredibly rare mineral, occurs naturally. Synthetic SiC powder has been mass-produced as an abrasive since 1893.
2. What is the Polar covalent bond?
A polar covalent bond is a kind of covalent bond that lies between pure covalent bonds and ionic bonds. Such bonds are formed when the difference in electronegativity between the anion and the cation is between 0.4 and 1.7, they form.
3. What is electron affinity?
The amount of energy released when an electron is added to a neutral atom to produce an anion is known as electron affinity. When an electron is added to a neutral gaseous atom to generate a negative ion, the potential energy of the atom changes.
4. Is BF3 Polar or Nonpolar?
BF3 is a non-polar compound. In BF3, the central boron atom has sp2 hybridized orbitals, resulting in an unfilled p orbital on the Bron atom and trigonal planar molecular geometry. Because the Boron-Fluorine bonds are all 120 degrees apart, any net dipole in that plane is canceled out. Even if each B-F bond is polar, the net dipole moment is zero because adding the bond vectors cancels everything out.
Check out the full article “Is BF3 polar or nonpolar?”.
5. Is MgCl2 Ionic or Covalent?
When the magnesium atom loses two electrons to create the Mg2+ ion and each chlorine receives one electron to form the Cl– ion, an ionic connection is formed between the magnesium and chlorine atoms.
Check “Is MgCl2 ionic or covalent?”.
6. Is SiCl4 polar or nonpolar?
SiCl4 (silicon tetrachloride) is nonpolar due to its symmetrical structure.
The center silicon atom is bonded to four Chlorine atoms.
Each Si-Cl bond is polar because the chlorine (3.16) atom is more electronegative than the silicon (1.90) atom.
However, in terms of the molecule’s dipole moment, the four single bonds between the Si-Cl atoms behave in opposite directions.
As a result, the net dipole moment becomes zero, indicating that this combination has no poles. As a result, SiCl4 is considered a nonpolar molecule.
7. NH3 is a polar or nonpolar molecule?
Ammonia (NH3) is a polar molecule. Since the molecule contains three N-H bond dipoles that do not cancel out. In each bond, nitrogen is more electronegative than hydrogen. This unequal distribution of charges among both nitrogen and hydrogen atoms makes NH3 a polar molecule.
8. CH4 polar or nonpolar?
Methane (CH4) is a nonpolar molecule.
More Interesting Topics
H2S Polar Or Nonpolar
SO2 Polar or Nonpolar
Is BF3 Polar or Nonpolar?
Sulfurous Acid Formula & Lewis Structure
Is O2 Polar or Nonpolar?| Easy Explanation
Is MgCl2 Ionic or Covalent?
Is HCl Polar or Nonpolar?| Simple Explanation
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023



