The number of particles present in one mole of a material is known as Avogadro’s number or Avogadro’s constant. It is the number of atoms in 12 grams of a carbon atom. This number was established experimentally to be around 6.0221 x 1023 particles per mole. The symbol L or NA can be used to represent Avogadro’s number. It is worth noting that Avogadro’s number is a dimensionless quantity on its own.
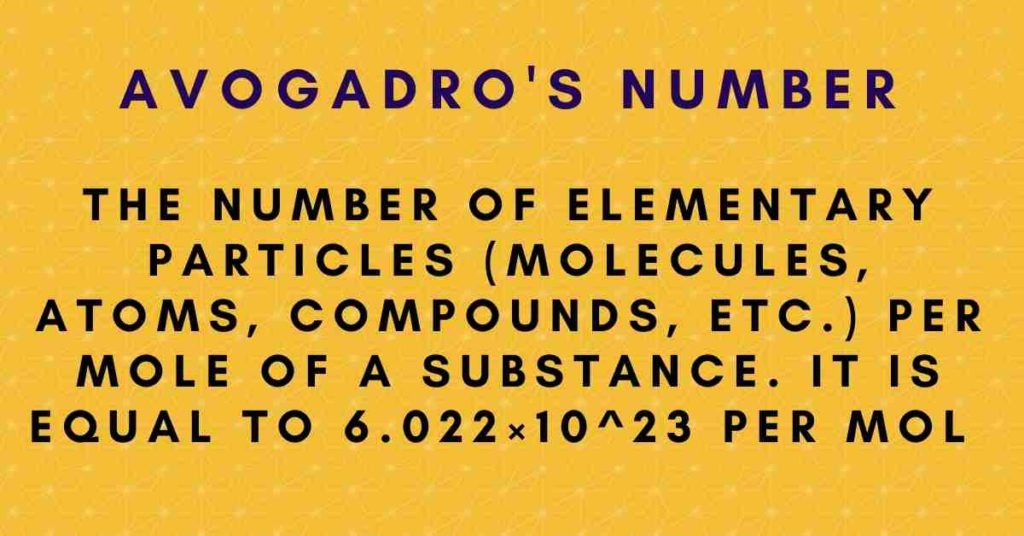
Table of Contents
What is a Mole?
In chemistry, a mole is a standard scientific unit for measuring significant amounts of extremely small things such as atoms, molecules, or other defined particles. It is also written mol.
The mole indicates a large number of units, 6.02214076 x 1023.
The mole was established by the General Conference on Weights and Measures as this quantity for the International System of Units (SI) effective May 20, 2019.
Why Avogadro’s number is important?
Avogadro’s number is one of the fundamental constants of chemistry. It allows one to compare various atoms or molecules of a particular material when the same number of atoms or molecules are being compared.
More Interesting Links
Sulfurous Acid Formula & Lewis Structure
Silicon Carbide – An Overview
Sulfur Electron Configuration
Fluoroantimonic acid-Super Acid
CH4 Polarity
Hydrogen Bond| Definition & Easy Explanation
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023