In the N2O Lewis structure, nitrogen (N) and oxygen (O) atoms are covalently bonded. The number of valence electrons in N and O is five and six, respectively. The total number of valence electrons in N2O is 16. N2O or nitrous oxide is commonly known as a laughing gas. Also, several other names by which this compound is known, such as sweet air, protoxide of Nitrogen, etc.
| Name of molecule | Nitrous oxide (N2O) |
| Bond Angles | 180 degrees |
| Molecular Geometry of N2O | Linear |
| No of Valence Electrons in the molecule | 16 |
| The dipole moment of N2O | 0.160880 D [Reference] |
Table of Contents
N2O Lewis Structure (Step by Step Construction)
In the N2O Lewis structure, the overall ratio of Nitrogen to Oxygen atom is 2:1. The following are the steps to construct the Lewis Structure.
Step-1: Count the valence electrons of atoms.
For the N2O Lewis structure, we need to figure out the number of valence electrons in individual atoms as shown in the table.
| Atom | Electronic Configuration | Valence Electrons (VEs) |
| 8O | 1S2 2S2 2P4 | 6 |
| 7N | 1S2 2S2 2P3. | 5 |
Valence electrons (VEs) in Nitrous Oxide = VEs in Oxygen atom + VEs in Nitrogen atoms
= 1(6)+2(5)
Valence electrons = 16 electrons.
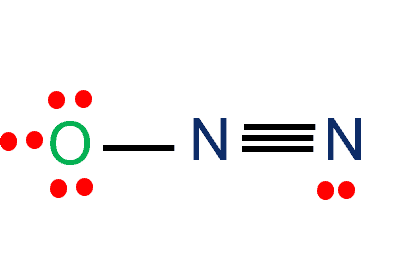
Step-2: Determine the central atom
As per rule, N is placed in the middle because it’s less electronegative than Oxygen.
If we check the proper arrangement of N and Oxygen in the periodic table, we will find that the electronegativity of N is 3.0 and the electronegativity of O is 3.5.
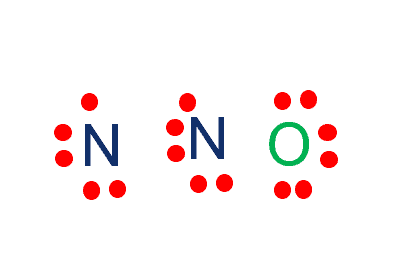
Step-3: Place electron pairs between the atoms
We need to distribute the 16 remaining valence electrons. First, we will draw a skeletal structure with single bonds only.
Out of 16 electrons, 4 will be used in pairs between atoms. Now we have 12 valence electrons to distribute. Nitrogen with 5 valence electrons needs 3 more electrons to fill the octet whereas Oxygen with 6 valence electrons needs 2 more electrons to complete the octet.
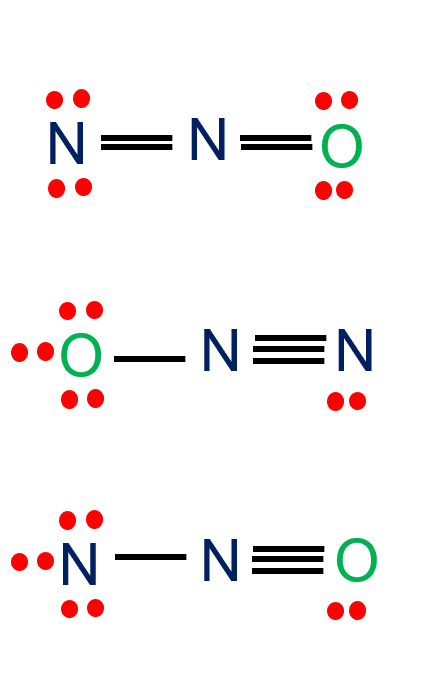
Step 4: Complete Octet
Give multiple bonds if required for fulfilling the octet of the atoms. At last, make sure all the atoms are having their lowest possible formal charge. The figure at the right shows three possible Lewis structures.
What is Nitrous Oxide
Nitrous oxide (N2O), is also known as laughing gas. It is important for a variety of medical applications because of its anesthetic use. It is insoluble in water and works as a powerful oxidizer at higher temperatures. It’s a colorless gas with a slightly sweet odor. It can cause a narcotic effect at higher concentrations.
Laughing Gas
Nitrous oxide is a colorless, non-flammable gas. It’s also frequently referred to as “laughing gas” or “happy gas.” Hence, if a mixture of nitrogen oxide with a little oxygen is inhaled for a sufficiently long time, it produces hysterical laughter. Hence, nitrogen oxide is also known as “laughing gas”.
As a sedative, this gas is utilized in medical and dental operations. It assists in reducing anxiety and allows the patient to rest before the surgery.
You may open this link to laugh without laughing gas.
Molar Mass of Nitrous oxide
The molar mass of Oxygen =16.00 g/mol.
Nitrogen molar mass = 14.01 x 2 = 28.02 g/mol.
The molar mass of Nitrous oxide = 44.013 g/mol
N2O Lewis Structure – Key Points

- The simplest formula for Nitrous oxide is N2O
- It is a famous inhaled anesthetic as it works as a quick pain reliever.
- Molar mass of N2O is 44.013 g/mol
- Nitrous oxide, from being used as an oxidizer in a rocket motor to its usage in internal combustion engines, has immense use in different fields. It is also used in aerosol propellants.
- As per the N2O Lewis structure, the molecular geometry of N2O is linear.
- It is used in the manufacturing of semiconductors
- Number of electrons in the valence shell of nitrogen atom = 5
- Number of electrons in the valence shell of oxygen atom = 6
- The charge of the central nitrogen atom is +1 in the N2O molecule
N2O Formal Charge
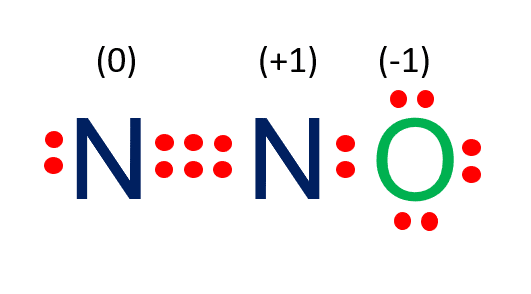
Formal charge = valence electrons – unbonded electrons – 0.5 bonded electrons
Valence electrons in Nitrogen = 5 electrons
Unbonded electrons in Nitrogen = 2 electrons
Half of the bonded electrons in Nitrogen =6/2 =3 electrons
Formal charge on Nitrogen = 5 – 2 – 3 = 0
Valence electrons in central Nitrogen = 5 electrons
Unbonded electrons in central Nitrogen = 0 electrons
Half of the bonded electrons in central Nitrogen =8/2 =4 electrons
Formal charge on central Nitrogen =5 – 0 – 4 =1 electron
Valence electrons in Oxygen = 6 electrons
Unbonded electrons in oxygen = 6 electrons
Half of the bonded electrons in oxygen are =2/2 =2 electrons.
Formal charge on oxygen = 6-6-1 = -1 electrons.

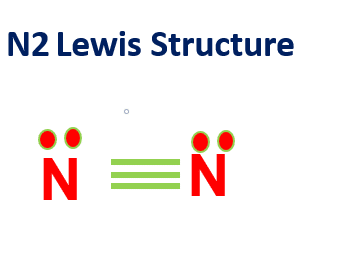
N2 Lewis Structure
N2 Lewis structure would comprise two atoms of nitrogen (N) atoms. There is a triple bond between both nitrogen atoms.
Each N is surrounded by two dots, which are called lone pairs of electrons.
These lone pairs (unbonded electrons) represent another 6 electrons in the N2 triple bond.
Therefore, in the N2 Lewis structure, each nitrogen atom is surrounded by 8 total valence electrons, giving it an octet and making it stable.
The molecular structure shows that the N2 molecule is perfectly symmetric.
Therefore, we can conclude that N2 is a nonpolar substance.
Related Links
CO2 Lewis Structure and Molecular Geometry
HCN Lewis Structure| Step By Step Construction
SiO2 Lewis Structure| Step By Step Construction
SO2 (Sulfur Dioxide) Lewis structure
NH3 Lewis Structure & Molecular Geometry
What is the Molar Mass of Nitrogen?
Frequently Asked Questions (FAQs)
Nitrous oxide is a neutral oxide. It does not form salt when reacted with acids or bases.
If a mixture of Nitrous Oxide with little Oxygen is inhaled for a sufficiently long time, it produces hysterical laughter.
Lewis structure of N2O helps us to find out about the structure of the compound, its types, the number of bonds, physical properties, and how the compound interacts with other compounds.
More Lewis Structure
CH4 Lewis Structure & Molecular Geometry
O2 Lewis Structure & Molecular Geometry
H2S Lewis Structure & Molecular Geometry
SO2 Lewis Structure| 4 Simple Steps
O2 Lewis Structure & Molecular Geometry
Author
Umair Javed
Umair has been working at Whatsinsight since 2020 as a content writer.
He has a Masters degree in Materials Science.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023



