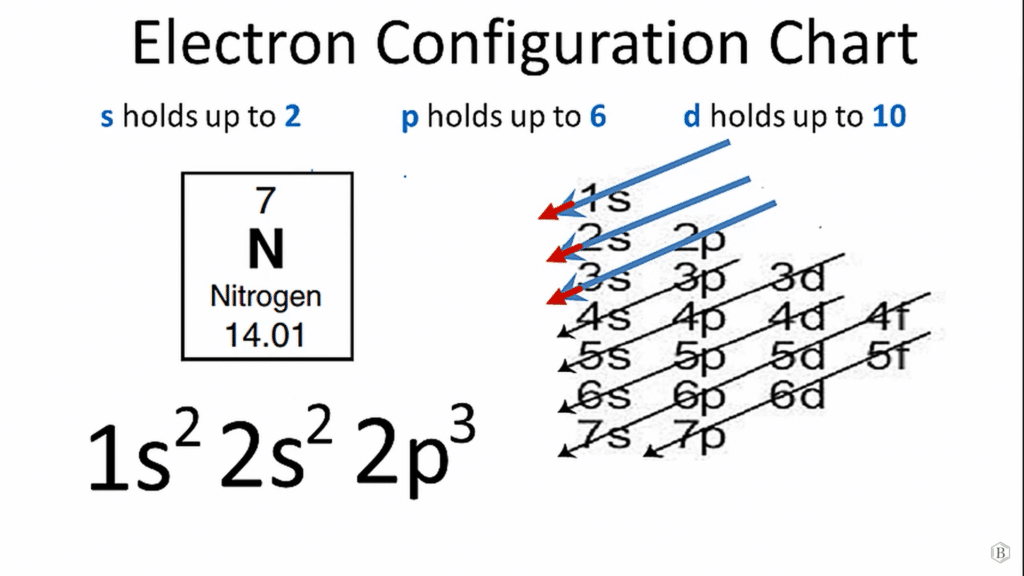
Nitrogen has 5 valence electrons. Nitrogen (N) is a non-metallic element in Periodic Group 15 [Va]. It is a colorless, odorless, and tasteless gas that is the most abundant element in the Earth’s atmosphere and is found in all living things. The mass number of nitrogen is 14, and its atomic number is 7. Since the 1s orbital can only hold two electrons, the next two electrons for N go into the 2s orbital. The remaining three electrons will go into the 2p orbital. Therefore, the electronic configuration of nitrogen is 1s22s22p3 .
Nitrogen is a nonmetal with 5 electrons in its valence shell, so it must combine with 3 hydrogen atoms to fulfill the octet rule and form ammonia, a stable compound (NH3). This results in the formation of two electrons that cannot be used for bonding (otherwise nitrogen would have to share more than 8 electrons, which is impossible). These electrons are paired and unshared in the ammonia molecule, indicating that they are not involved in bonding. These types of electron pairs are known as lone pairs, unshared electrons, or nonbonding electrons.
| Name of molecule | Nitrogen |
| Bond Angles | 180 degrees |
| Molecular Geometry of Nitrogen | Linear |
| The polarity of the N2 molecule | Nonpolar |
| N2 valence electrons | 10 |
| The molar mass of nitrogen | 28 atomic mass units |
| Nitrogen valence electrons | 5 |
Table of Contents
Valence Electrons
Valence electrons are a single outer shell electron in an atom’s outer shell that is responsible for the atom’s chemical characteristics. In other words, a valence electron is an atom’s electron that may be transferred to or shared with another atom and is placed in the atom’s outermost shell.
Summary
- Nitrogen is a nonmetal with a mass number of 14 and an atomic number of 7.
- Nitrogen has five valence electrons.
- The electronic configuration of nitrogen is 1s22s22p3 .
- In a nitrogen molecule, a triple covalent bond is represented by three lines between two atoms of nitrogen.
- The bond angle is 180 degrees, and there are 10 valence electrons.
- N2 is a nonpolar molecule with linear geometry.
- The molar mass of nitrogen is 28 atomic mass units (AMU).
Related Links
How many electrons does oxygen have?
SiO2 Lewis Structure| Step By Step Construction
What is the molar mass for sulfur dioxide?
What is the Molar Mass of Nitrogen?
Frequently Asked Questions
1. Is titanium magnetic?
Titanium, being a paramagnetic substance, is only weakly attracted to magnets. The presence of four unpaired electrons in its electrical structure is the primary cause of its paramagnetic characteristics (check full article is titanium magnetic?).
2. Is aluminum magnetic?
Aluminum (United States English), also known as Aluminum (British English), is not magnetic under normal conditions, owing to its crystal structure. Along with other metals like magnesium and lithium, it’s classified as a paramagnetic substance.
In other words, Aluminum is not magnetic; nevertheless, in the presence of an external magnetic field, Aluminum becomes “slightly” magnetic as its electron aligns with the magnetic field.
3. What is the density of water g/ml?
This density of water is equal to a rounded value of 1 gram per milliliter (g/ml) or 1 gram per cubic centimeter (g/cm3). (Check the full article to know the accurate value of density of water in g/ml)
4. How many neutrons are in hydrogen?
The vast majority of hydrogen atoms lack neutrons, making them the lightest atoms possible with only one electron and one proton (check full article How Many Neutrons Does Hydrogen Have?)
5. What is the brf3 bond angle?
BrF3 bond angle is 86.20. Bromine trifluoride (BrF3) is a pale yellow interhalogen liquid with a pungent odor. Although sulfuric acid interacts intensely with water and organic molecules, it dissolves it. It’s an inorganic ionizing solvent (check the full article to know more about it brf3 bond angle).
6. How many electrons does iron have?
Iron contains eight valence electrons. Because iron is a transition metal, electrons in its d subshells can be used as valence electrons. In a transition metal, valence electrons are electrons that exist outside of a noble-gas core. Iron has the electrical configuration 1s22s22p63s23p64s23d6.
7. What’s the molar mass of Aluminum?
Aluminum molar mass is 26.982 g/mol.
10. How many electrons does oxygen have?
A single oxygen atom has eight protons, eight electrons, and eight neutrons.
Oxygen is a stable isotope of oxygen with a nucleus of 8 neutrons and 8 protons. Its mass is 15.99491461956 u. Check full topic “How many electrons does oxygen have?”.
11. What is nitrogen fluoride?
Nitrogen trifluoride (NF3) is a colorless gas that has a moldy odor. It is extremely toxic when inhaled.
12. How cold is liquid nitrogen?
Liquid nitrogen is nothing more than extremely cold nitrogen. The temperature is 320 degrees Fahrenheit below zero (-196oC). It’s so cold that anything it comes into contact with instantly freezes.
Check the full article here“How cold is liquid nitrogen?”.
13. What is calcium electronic configuration?
Calcium electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2
14. How many electrons does Helium have?
A single helium atom has two protons, two electrons, and two neutrons. The second element in the periodic table is helium. Because s subshells may store up to two electrons, both electrons fit into the 1s subshell, resulting in the electron configuration for helium atoms being 1s2.
More Interesting Topics
is b2 paramagnetic or diamagnetic?
NH3 Lewis Structure & Molecular Geometry
Is Nh3 Polar?
N2O Lewis Structure| Nitrous oxide-Laughing Gas
HCN Lewis Structure & Molecular Geometry
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023



