Phosphorus trifluoride, often abbreviated as PF3 or PF₃, is a chemical compound composed of phosphorus (P) and three fluorine (F) atoms. In this comprehensive guide, we will unveil the Lewis structure of PF3 and delve into its molecular shape, bond characteristics, electronegativity, bond angles, and other key properties.
| Name of Molecule | Phosphorus Trifluoride (PF₃) |
| Bond Angles of PF3 | approx. 107degrees |
| Molecular Geometry of PF3 | trigonal pyramidal |
| No. of Valence Electrons | 26 |
| Hybridization | sp3 |
| Polarity | polar |
| Bond Lengths | 1.54 to 1.57 angstroms (Å) |
Table of Contents
What is the Lewis Structure for PF3 and How to Draw It
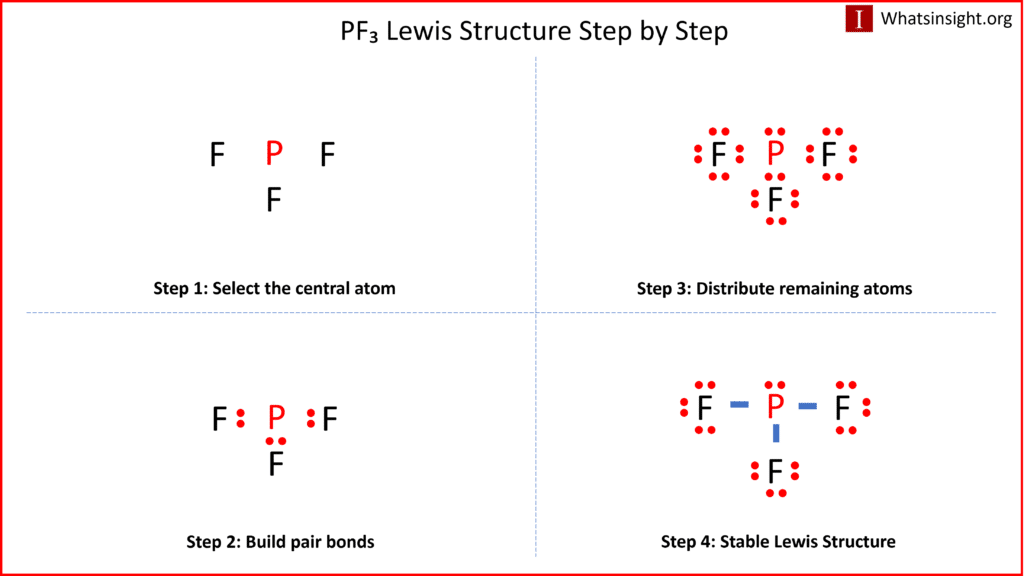
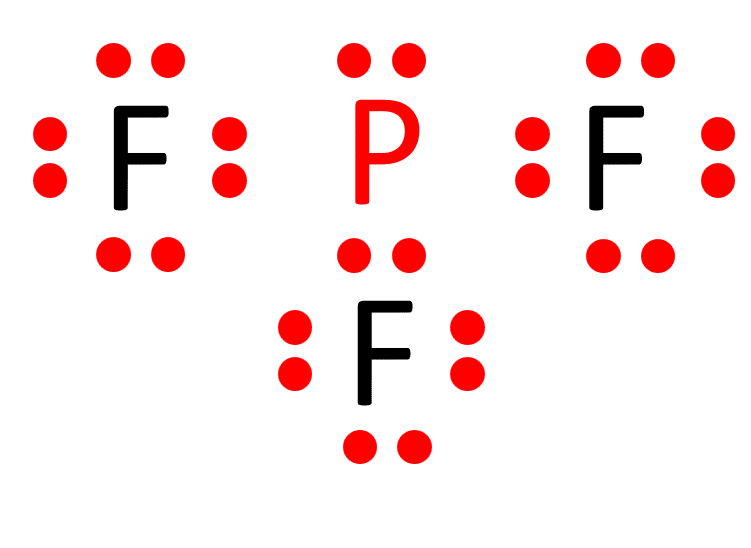
The Lewis structure of a molecule provides a visual representation of its atom arrangement and valence electrons. To draw the Lewis structure for PF3, follow these steps:
a. Calculate the total number of valence electrons in PF3: Phosphorus (P) has 5 valence electrons, and each fluorine (F) atom possesses 7 valence electrons. With three fluorine atoms in PF3, the total valence electrons sum up to 26 valence electrons.
b. Determine the central atom. In PF3, phosphorus acts as the central atom due to its relatively lower electronegativity compared to fluorine. Electronegativity is a measure of an atom’s tendency to attract electrons. Therefore, placing fluorine in the center would result in a less stable structure.
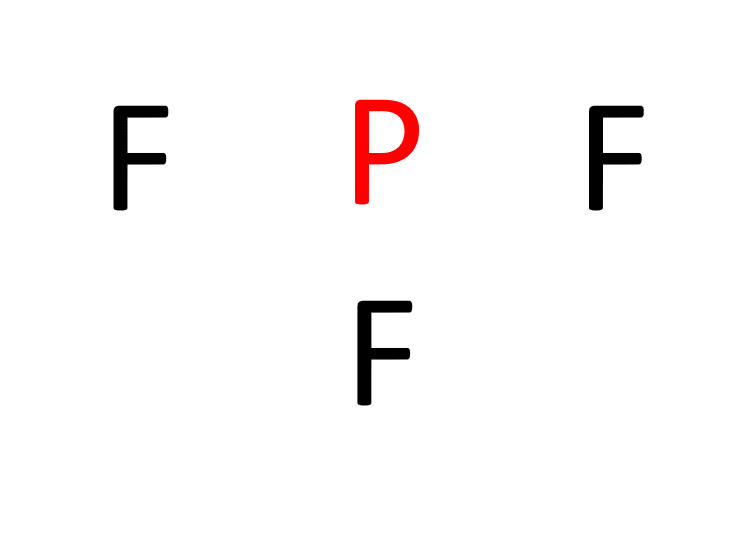
c. Establish single bonds connecting the atoms. Phosphorus forms single bonds with each of the three fluorine atoms (P-F).
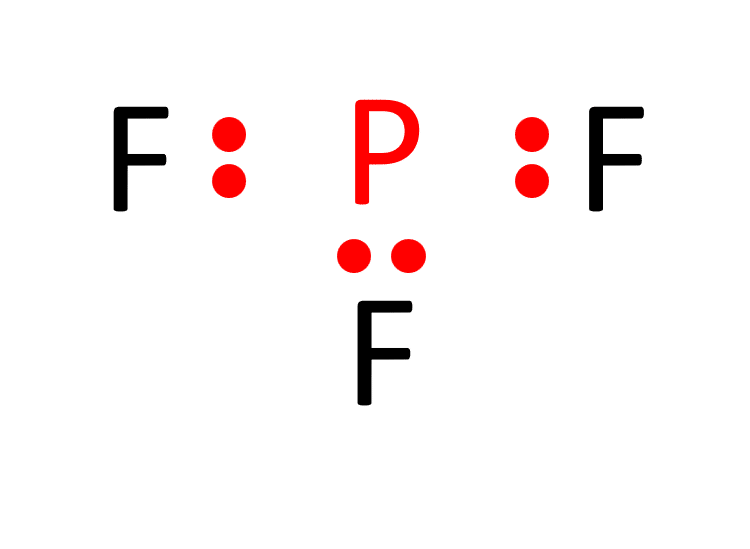
d. Distribute the remaining valence electrons to satisfy the octet rule. Each fluorine atom attains a full valence shell by forming a single bond with phosphorus, resulting in the Lewis structure of PF3.
Properties of PF3
PF3 is a colorless and odorless gas under standard conditions. It is relatively stable and serves as a valuable ligand in coordination chemistry, forming complexes with various metal ions. Additionally, PF3 can act as a reducing agent in certain chemical reactions.
Molecular Shape of PF3
The molecular shape of PF3 is trigonal pyramidal. In this configuration, the central phosphorus atom is bonded to three fluorine atoms, and an unshared pair of electrons on phosphorus creates a pyramidal shape.
Bond Type in PF3
In PF3, phosphorus forms single bonds with each of the three fluorine atoms. These are sigma (σ) bonds, resulting from the overlap of atomic orbitals.
Bond Angles in PF3
The bond angles between the central phosphorus atom and the three fluorine atoms in PF3 are approximately 107 degrees. This specific angle results from the repulsion of electron pairs around the central atom, leading to the characteristic trigonal pyramidal shape.
Bond Lengths in PF3
The bond lengths in PF3, specifically the P-F bond lengths, are relatively uniform. They typically fall within the range of 1.54 to 1.57 angstroms (Å). The consistency in bond lengths contributes to the stability of the molecule.
Hybridization in PF3
Phosphorus in PF3 undergoes sp3 hybridization, involving the combination of one s orbital and three p orbitals of phosphorus to form four sp3 hybrid orbitals. These hybrid orbitals overlap with the p orbitals of fluorine atoms, creating sigma bonds and maintaining the trigonal pyramidal molecular shape of PF3.
Conclusion
In conclusion, understanding the Lewis structure, molecular shape, bond characteristics, electronegativity, bond angles, and properties of PF3 provides profound insights into its chemical behavior and significance in various applications. The choice of phosphorus as the central atom, bond angles, and bond lengths all contribute to the unique properties and reactivity of this compound.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023