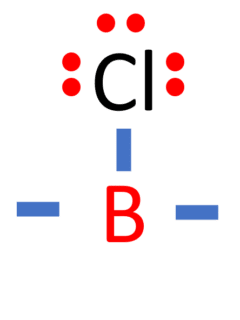
Boron trichloride, commonly abbreviated as BCl3 or BCl₃, is a chemical compound composed of boron (B) and three chlorine (Cl) atoms. In this comprehensive guide, we will unveil the Lewis structure of BCl3 and delve into its molecular shape, bond characteristics, electronegativity, bond angles, and other key properties. Name of Molecule Boron trichloride (BCl₃) Bond …
Umair Javaid
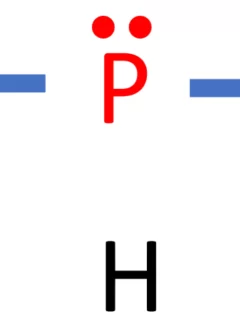
Phosphine, represented as PH3 or PH₃, is a chemical compound composed of one phosphorus (P) atom bonded to three hydrogen (H) atoms. In this comprehensive guide, we will unveil the Lewis structure of PH3 and delve into its molecular shape, bond characteristics, properties, and its significance in the field of chemistry. Name of Molecule PH₃ …
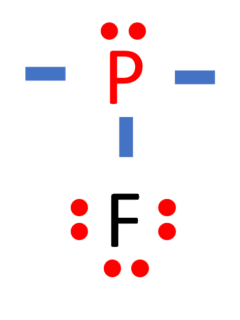
Phosphorus trifluoride, often abbreviated as PF3 or PF₃, is a chemical compound composed of phosphorus (P) and three fluorine (F) atoms. In this comprehensive guide, we will unveil the Lewis structure of PF3 and delve into its molecular shape, bond characteristics, electronegativity, bond angles, and other key properties. Name of Molecule Phosphorus Trifluoride (PF₃) Bond …
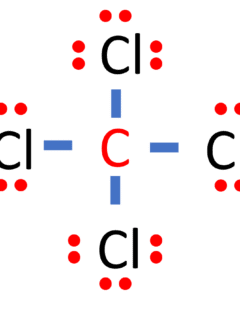
Carbon tetrachloride, often abbreviated as CCl4 or CCl₄, is a chemical compound composed of one carbon (C) atom bonded to four chlorine (Cl) atoms. In this comprehensive guide, we will unveil the Lewis structure of CCl4 and delve into its molecular shape, bond characteristics, properties, historical uses, and environmental considerations. Name of Molecule Carbon Tetrachloride …
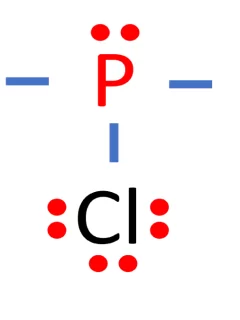
Phosphorus trichloride, often abbreviated as PCl3 or PCl₃, is a chemical compound composed of phosphorus (P) and chlorine (Cl) atoms. In this comprehensive guide, we will unveil the Lewis structure of PCl3 and delve into its molecular shape, bond characteristics, polarity, and hybridization. Name of Molecule Phosphorus Trichloride (PCl3) Bond Angles approx. 109.5 degrees Molecular …
Carbon disulfide, often denoted as CS2 or CS₂, is a chemical compound consisting of carbon (C) and sulfur (S) atoms. In this comprehensive guide, we will unravel the Lewis structure of CS2 in four simple steps and delve into its molecular shape, bond characteristics, polarity, and hybridization. Name of Molecule Carbon Disulfide Bond Angles 180 …
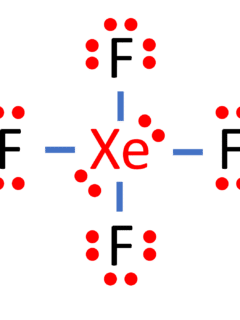
Xenon Tetrafluoride, represented as XeF4, is a fascinating chemical compound that has garnered significant attention in the world of chemistry due to its intriguing molecular structure and unique properties. In this article, we will embark on an in-depth exploration of the Lewis structure of XeF4, discussing its valence electrons, molecular geometry, hybridization, and polarity. Formula …
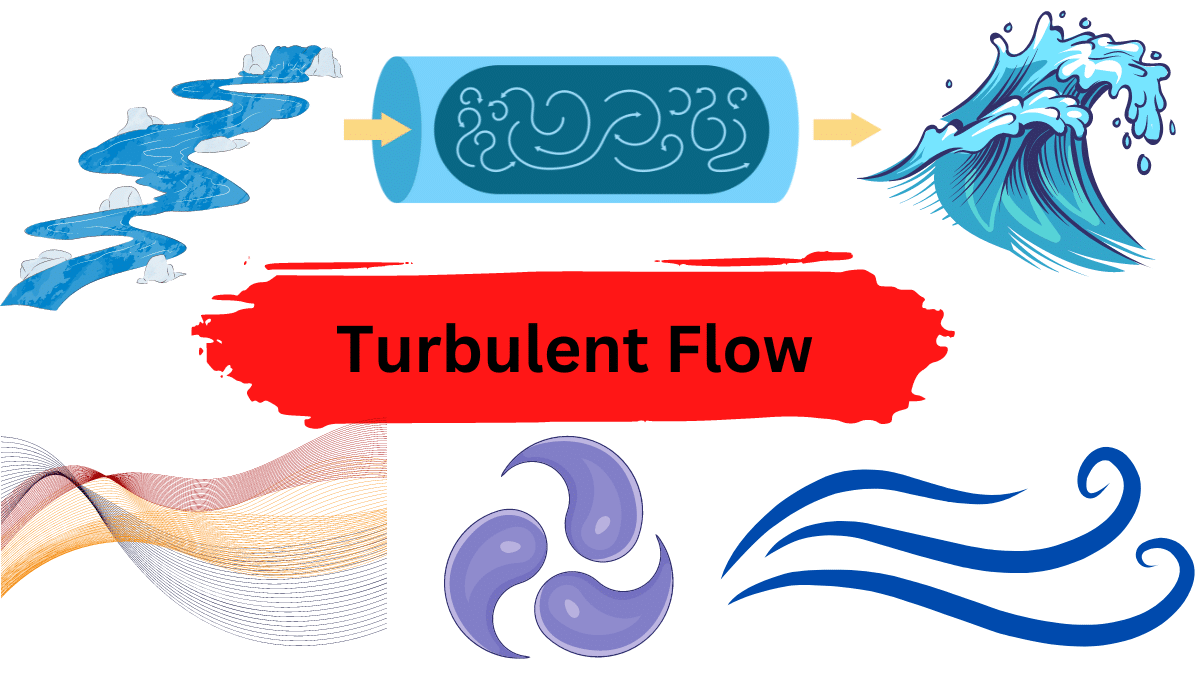
Turbulent flow refers to a dynamic pattern of fluid (gas or liquid) movement characterized by erratic fluctuations and mixing, in contrast to the orderly motion observed in laminar flow. In turbulent flow, the fluid undergoes constant alterations in both speed and direction at a particular point. This phenomenon is commonly observed in the movement of …
Radioactive decay is a fascinating natural phenomenon that lies at the heart of nuclear physics. It involves the spontaneous transformation of unstable atomic nuclei into more stable configurations, accompanied by the emission of various types of radiation. This topic provides a comprehensive overview of radioactive decay, exploring its definition, mechanisms, and key concepts. Definition of …
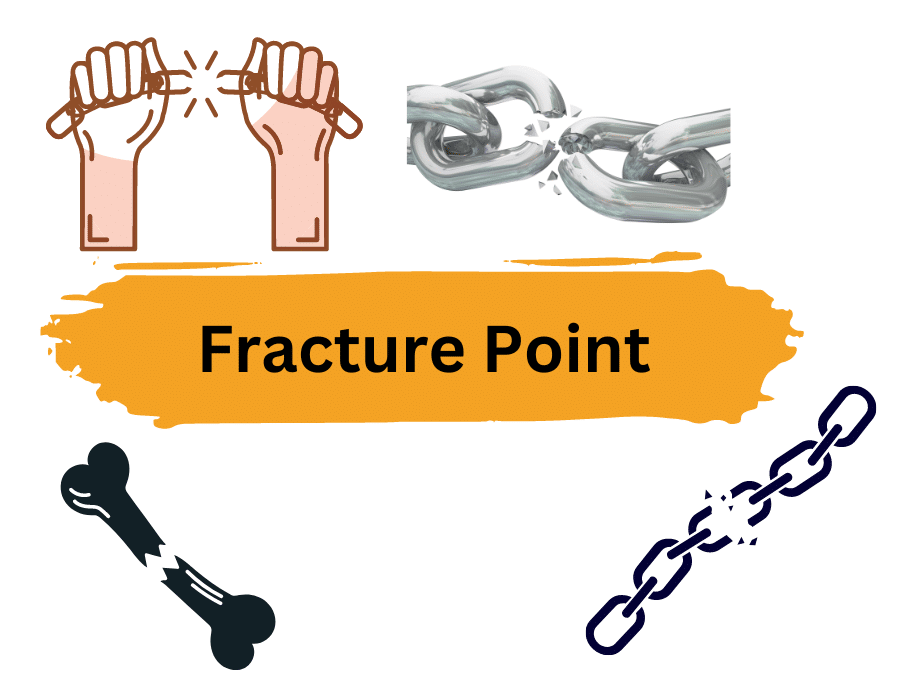
The fracture point is the point where a material breaks apart due to strain. It’s the moment when the strain on the material reaches its highest value, causing it to physically separate. This can happen even if the corresponding stress on the material is lower than its ultimate strength. Ductile materials have a fracture strength …
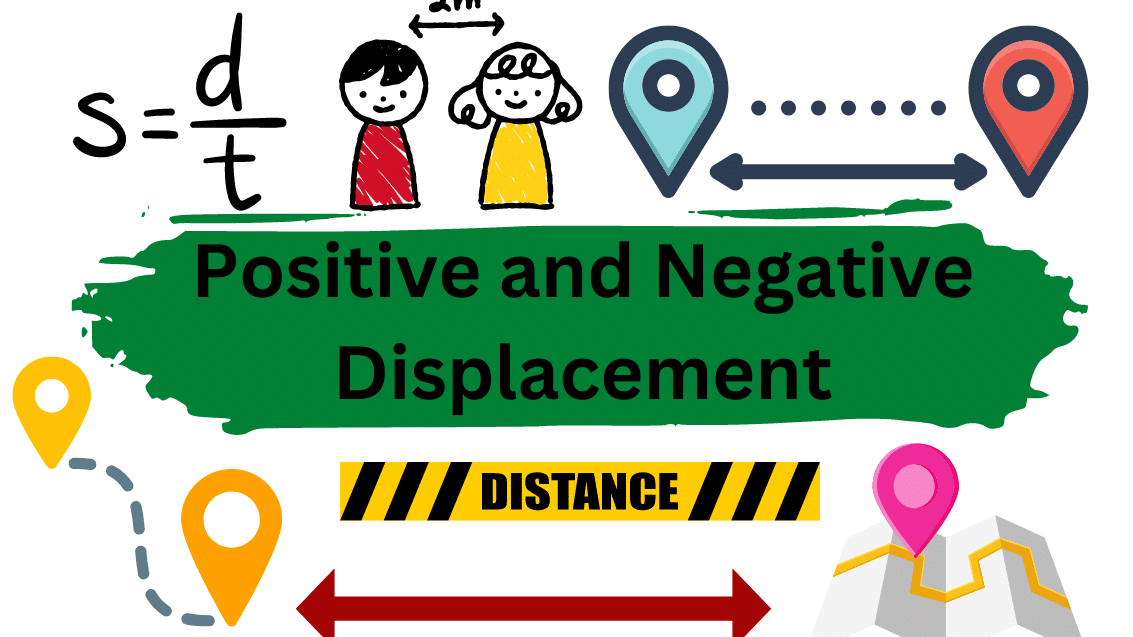
Positive and negative displacements refer to the direction of motion or movement of an object relative to a reference point. Positive displacement occurs when an object moves in the same direction as the reference point. For example, if a person walks 5 meters forward from a starting point, their displacement would be positive (+5 meters) …
Viscous force, in simple terms, refers to the resistance experienced by an object when it moves through a fluid (liquid or gas). This force is caused by the internal friction between different layers of the fluid. The formula to calculate viscous force is given by: Viscous Force = Coefficient of Viscosity × Area × Velocity …
Dynamic pressure (sometimes called velocity pressure) refers to the force exerted by a moving fluid, such as air or water, on an object in its path. It is a measure of the impact and energy transferred by the fluid due to its motion. In simpler words, dynamic pressure can be thought of as the “push” or …
Mechanical stress and strain are fundamental concepts in the field of materials science and engineering that describe how materials respond to external forces. Mechanical stress refers to the internal forces or pressure exerted on a material when an external force is applied. It is defined as the force per unit area acting on a material. …
Coulomb’s Law is all about how charged things (like balloons or socks rubbed on carpet) attract or repel each other. It says that the amount of attraction or repulsion depends on two things: how much charge each object has, and how far apart they are. Imagine two magnets – if you hold them close together, …