Nitric acid (HNO3) is a colorless, fuming, and extremely corrosive liquid that is used as a laboratory reagent and a significant industrial chemical in the production of fertilizers and explosives. It is poisonous and can cause serious burns.
In addition, although nitric acid is colorless while pure, but takes on a yellowish appearance as it ages owing to the accumulation of nitrogen oxides.
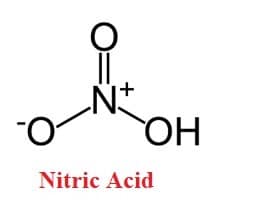
As shown nitric acid structure in the figure, Nitric acid is a nitrogen oxoacid with the formula HNO3, in which the nitrogen atom is bonded to a hydroxy group and to the remaining two oxygen atoms through equivalent bonds.
| Compound Name | Nitric acid (HNO3) |
| Other names | Hydrogen nitrate, Aqua fortis, Azotic acid |
| Color | Colorless liquid |
| Molar mass | 63.01 g/mol |
| Density | 1.51 g/cm³ |
| Odor | acrid odor |
Table of Contents
Important Facts-Nitric Acid
| Inorganic acids |
| A bitter taste |
| extremely corrosive and toxic. |
| Strong oxidizing agents |
| A good conductor of electricity |
| reacts with metals, oxides, and hydroxides, forming nitrate salts. |
| toxic and can cause severe burns. |
| key chemicals in the manufacture of fertilizers. |

Production of Nitric Acid
Commercial grade nitric acid solutions typically contain between 52 and 68 percent nitric acid. The Ostwald procedure, named after German scientist Wilhelm Ostwald, is used to produce nitric acid. Anhydrous ammonia is oxidized to nitric oxide in the presence of a platinum or rhodium gauge catalyst at a high temperature of around 500K and a pressure of 9 bar in this procedure.
Key Points

- Nitric acid is manufactured from ammonia and is an important component in the production of fertilizers.
- Conjugate acid of a nitrate.
- very toxic by inhalation. corrosive to metals or tissue.
- Long-term exposure to low concentrations or short-term exposure to high concentrations may have negative health consequences.
- Upon distillation, nitric acid in its pure form begins to boil at 78.2°C and becomes solid when it is well cooled.
Molar Mass of Nitric Acid
The molar mass of Oxygen =15.9994 g/mol.
Hydrogen molar mass = 1.00794 g/mol.
The molar mass of Nitrogen = 14.01 grams/mole
Molar mass of HNO3 = 63.01 g/mol
Uses of Nitric Acid
Nitric acid is utilized in the production of inorganic and organic nitrates and nitro compounds, which are employed in the production of fertilizers, color intermediates, explosives, and a variety of organic chemicals.
Is Nitric acid a strong acid?
Nitric acid is a strong acid. In an aqueous solution, nitric acid is totally ionized into hydronium (H3O+) and nitrate (NO3-) ions and is a potent oxidizing agent.

Important Links
- Nitric acid – SAFETY DATA SHEET
- MATERIAL SAFETY DATA SHEET
Related Links
Summary
Nitric acid (HNO3) is a very corrosive and hazardous strong acid that can result in serious burns. Aqua fortis is another name for it. Nitric acid is used in rocket fuels, to make the wood appear older, and in explosives.
Frequently Asked Questions
1. What are Acids?
An acid is a chemical that, when mixed with water, creates hydrogen (H+) ions. A hydrogen ion is made up of only one proton and no electron. When we examine the formulas of various acids, we can see that they all contain at least one H (hydrogen).
When we dissolve an acid molecule in water, it dissociates (breaks apart). Hydrochloric acid (HCl), for example, dissociates into hydrogen ions (H+) and chloride anions (Cl-).
Some examples of acid are:
- Hydrochloric acid
- Sulfuric acid
- Nitric acid
2. ka for HNO3
The Ka value of HNO3 is about 24. The Ka value, also known as the acid dissociation equilibrium constant, is a measure of the acidity of a solution. There are no units for these constants.
3. What is Base?
A basic is an acid-neutralizing chemical. When bases are added to water, they split to generate hydroxide ions, which are denoted by the symbol OH-. An alkaline solution is a base solution that has been added to water.
- NaOH – sodium hydroxide (caustic soda)
- NH4OH – solution of ammonia in water
- Ca(OH)2 – calcium hydroxide (builders’ lime)
4. What is Sulfur trioxide?
SO3 (sulfur trioxide) is a chemical compound. It is available in three forms: gaseous monomer, crystalline trimer, and solid polymer. It is solid at just below room temperature and has a relatively narrow liquid range. Gaseous SO3 is the primary precursor to acid rain.
5. NH3 is a polar or nonpolar molecule?
Ammonia (NH3) is a polar molecule. Since the molecule contains three N-H bond dipoles that do not cancel out. In each bond, nitrogen is more electronegative than hydrogen. This unequal distribution of charges among both nitrogen and hydrogen atoms makes NH3 a polar molecule.
6. CH4 polar or nonpolar?
Methane (CH4) is a nonpolar molecule. Nonpolar molecules form when electrons in a diatomic molecule are shared equally or when polar bonds in a bigger molecule cancel each other out.
7. Is SiCl4 polar or nonpolar?
SiCl4 (silicon tetrachloride) is a nonpolar molecule. Because the four chemical bonds between silicon and chlorine are uniformly distributed, SiCl4 is non-polar. A polar covalent bond is a type of covalent link that is intermediate between pure covalent bonds and ionic bonds. When the difference in electronegativity between the anion and the cation is between 0.4 and 1.7, such bonds occur.
Check the full article “Is SiCl4 polar or nonpolar?”.
8. What is nitrogen trifluoride gas?
Nitrogen trifluoride (NF3) is an odorless, colorless gas. When inhaled, it is exceedingly poisonous. Containers may burst violently if exposed to fire or heat for a lengthy period of time.
Author
Umair Javed
Umair has been working at Whatsinsight since 2020 as a content writer.
He has a Masters degree in Materials Science.
More Interesting Links
NH3 Lewis Structure & Molecular Geometry
Is NH3 Acid or Base?
Perchloric Acid| Is It Super Acid?
NH3 Molecular Geometry| Trigonal Pyramidal
NH3 Oxidation Number
Is Nh3 Polar?
Sulfurous Acid| Formula & Lewis Structure
Charge of Ammonia (NH3)| Simple Steps
Disclaimer
Whatsinsight.org‘s blog and everything published on it are provided solely as an information resource. The blog, its authors, and affiliates accept no responsibility for any accident, injury, or damage caused by following the information on this website, in part or in whole. We do not recommend using any chemical without first consulting the manufacturer’s Material Safety Data Sheet and following the safety advice and precautions on the product label.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023



