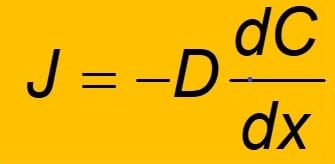A concentration gradient refers to the gradual change in the concentration of solutes.
Figure 1 shows the difference in the concentration of a substance between the two areas.
This article includes concentration gradient definition and examples, along with a brief introduction to diffusion and osmosis.
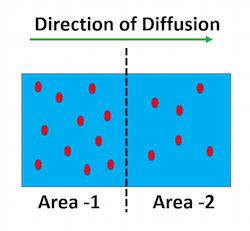
Table of Contents
Concentration Gradient Definition In Simple Words
The term “gradient” refers to the progressive increase or decrease of a variable with respect to distance.
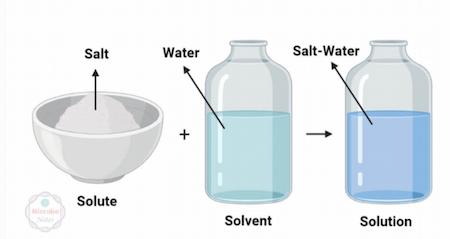
A solution, essentially, has two major components, the solvent, and the solute.
The solvent is the dissolving component, e.g., water.
The solute is the particles that are dissolvable by the solvent.
A concentration gradient is an outcome when the amounts of solutes between two solutions are different.
This imbalance of solutes between the two solutions drives solutes to move from a highly dense area to a less dense area.
This movement is an attempt to eliminate the imbalance of solute concentrations between the two solutions.
Diffusion & Concentration Gradient
Diffusion is the net transfer of molecules from a higher concentration area to a lower concentration area. This is due to the molecules’ spontaneous movement.
A concentration gradient is a difference in a substance’s quantity between two regions.
Diffusion Example

- The aroma of food diffuses into the air and reaches you.
- A tea bag placed in a cup of hot water will diffuse into the water.
- The smell of perfume spreading over a whole room is an example of diffusion.
- Steel can be diffused (e.g., with carbon or nitrogen) to modify its properties.
Examples of Concentration Gradient

- Ion gradients, such as Sodium/Potassium gradients
- When a membrane separates two different concentrations of molecules.
Gradient Definition
A gradient is a relationship between a change in one variable, such as concentration, pressure, or temperature, and a change in another variable, usually distance.
Some of the examples of the gradient are listed below:
- Concertation gradient: a change in concentration over a distance
- Pressure gradient: a change in pressure over a distance
- Temperature gradient: A change in temperature over a distance
Passive Transport
The process by which an ion or molecule moves through a cell wall via a concentration gradient, or from a high concentration to a low concentration, is known as passive transport or passive diffusion.
Osmosis
Water and other molecules move through a selectively permeable membrane during the osmosis process in order to balance the concentration of other substances.
The concentration difference and temperature have an impact on osmosis. The faster the rate of osmosis, the bigger the difference in concentration. The rate of osmosis also increases as the temperature rises.
Selectively Permeable Membranes
A selectively permeable cell membrane permits specific molecules or ions to pass through active or passive transport. Active transport mechanisms necessitate the use of cellular energy to move the materials, whereas passive transport does not necessitate the use of cellular energy.
Related Links
Density Of Water (g/ml)| Fresh Water and Sea Water
Mechanical Energy| 7 Daily Life Examples
Mass vs Weight| 5 Simple Examples
Centre of Mass and Centre of Gravity
Convection| Atmospheric Motions in the Vertical Direction
Summary
A concentration gradient occurs when the concentration of particles is higher in one region than in another. In passive transport, particles will diffuse along a concentration gradient, from higher concentrations to lower concentrations, until they are uniformly distributed.
Quick Quiz
a. The difference in the concentration of a substance from one region to another region is called
- Diffusion
- Conc gradient
- Passive transport
- osmosis
Answer: Option 2
b. Movement of molecules against their concentration gradient is called
- Diffusion
- Passive transport
- osmosis
Answer: Option 1: Diffusion.
More Interesting Links
Momentum Equation| Definition And Examples
Laminar Flow| Physics
Unit Weight of Water| Specific Weight
Kinematic Viscosity of Water
Is water a Pure Substance?
Frequently Asked Questions (FAQs)
1. What is Passive transport?
Passive transport is a type of membrane transport in which chemicals are moved across cell membranes without the need for energy. Passive transport relies on the second law of thermodynamics (difference in concentration between two regions) to drive the movement of substances across cell membranes rather than cellular energy, as active transport does.
2. What is Active transport?
Active transport is the movement of molecules across a cell membrane across a concentration gradient from a region of lower concentration to a region of higher concentration. To achieve this movement, active transport necessitates the use of cellular energy.
3. Why do we need Selective Permeability?
Cellular membranes have a trait called selective permeability that permits only particular molecules to enter or exit the cell. This is necessary for the cell to retain its internal order regardless of environmental changes.
4. What is the specific heat of air?
The specific heat of air at constant pressure, is 1.005 kJ/kg K and the specific heat of air at constant volume is 0.718 kJ/kg K. The specific heat (C), also called heat capacity, of a substance, is the amount of heat required to raise its temperature by one degree.
5. Combustion reaction?
Combustion is a chemical process that produces both heat and light. The most prevalent sort of combustion is fire. When the gas oxygen interacts with another material, the majority of combustion happens.
6. What is the sublimation process?
Sublimation is the process by which a solid turns straight into a gaseous state without first going through the liquid stage. Dry ice is one of several sublimation instances (frozen carbon dioxide).
7. The process of distillation?
Distillation is the separation of a mixture of liquids based on variations in their boiling points (or volatility). Water may be extracted from a salt solution using this method.
8. is hydrogen a metal?
Hydrogen is a nonmetal that is placed above the alkali metals in the periodic table due to its ns1 electron configuration.
9. Strain energy?
Strain energy is a type of potential energy stored in an item as a result of elastic deformation. When an item is deformed from its unstressed state, the external work done on it is turned into (and is considered equivalent to) the strain energy contained in it.
10. Gauge pressure?
Gauge pressure, often known as overpressure, is the pressure in a system that is greater than atmospheric pressure. Because gauge pressure is zero-referenced against ambient air (or atmospheric) pressure, gauge pressure values include atmospheric pressure.
11. Why do we need osmosis?
Osmosis occurs when water passes across a semipermeable barrier from a low solute concentration (low osmolarity) to a high solute concentration (high osmolarity).
It is one of the most important ways that plants and animals achieve homeostasis.
Osmosis is vital in the human body, particularly in the gastrointestinal and renal systems. Osmosis aids in the extraction of nutrients from meals. It also removes waste items from your bloodstream.
More Links
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023