Phosphine, represented as PH3 or PH₃, is a chemical compound composed of one phosphorus (P) atom bonded to three hydrogen (H) atoms. In this comprehensive guide, we will unveil the Lewis structure of PH3 and delve into its molecular shape, bond characteristics, properties, and its significance in the field of chemistry.
| Name of Molecule | PH₃ (Phosphine) |
| Bond Angles of PH3 | 93.5° |
| Molecular Geometry of PF3 | Trigonal Pyramidal |
| No. of Valence Electrons | 8 |
| Hybridization | sp3 |
| Polarity | Polar |
Table of Contents
What is the Lewis Structure for PH3 and How to Draw It:
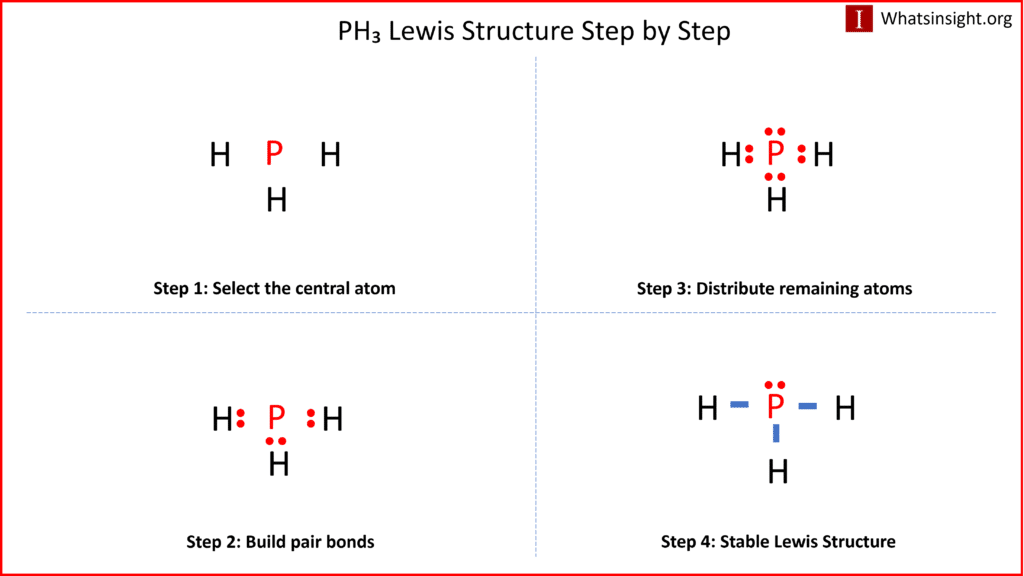
The Lewis structure of a molecule provides a visual representation of its atom arrangement and valence electrons. To draw the Lewis structure for PH3, follow these steps:
a. Calculate the total number of valence electrons in PH3: Phosphorus (P) has 5 valence electrons, and each hydrogen (H) atom possesses 1 valence electron. With three hydrogen atoms in PH3, the total valence electrons sum up to 5 (from phosphorus) + 1 x 3 (from hydrogen) = 8 valence electrons.
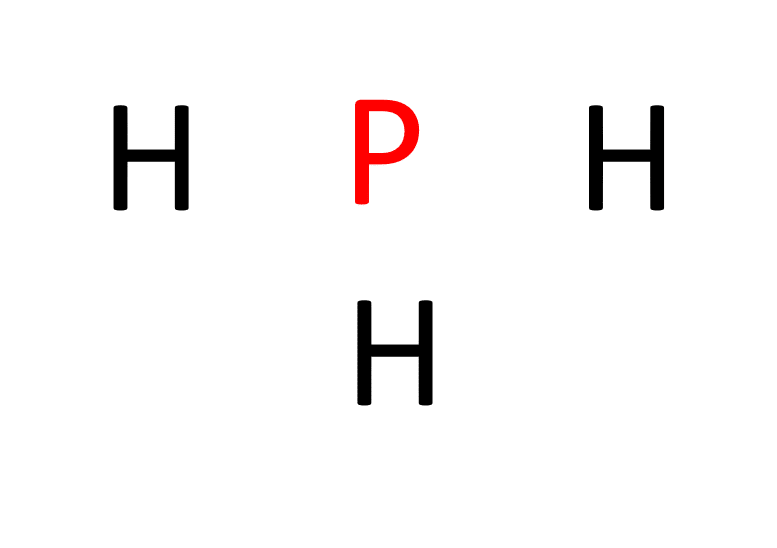
b. Determine the central atom. In PH3, phosphorus acts as the central atom because it can form more bonds than hydrogen.
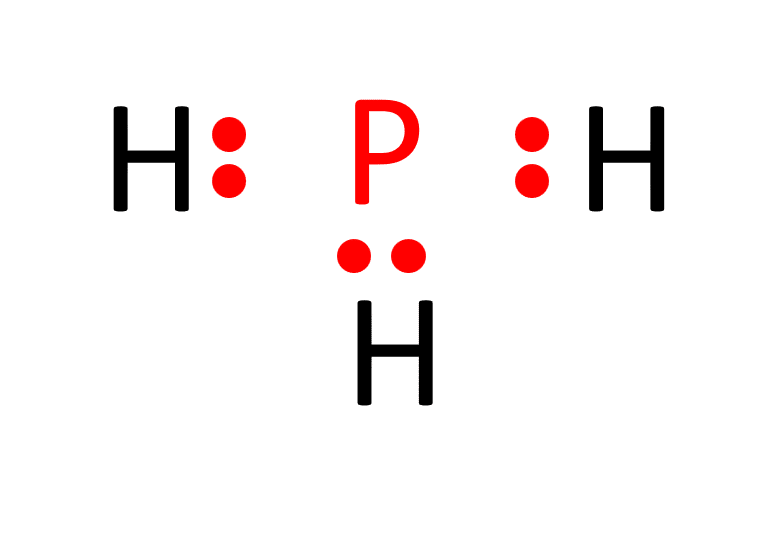
c. Establish single bonds connecting the atoms. Phosphorus forms single bonds with each of the three hydrogen atoms (P-H).
d. Distribute the remaining valence electrons to satisfy the octet rule. Each hydrogen atom attains a full valence shell by forming a single bond with phosphorus, resulting in the Lewis structure of PH3.
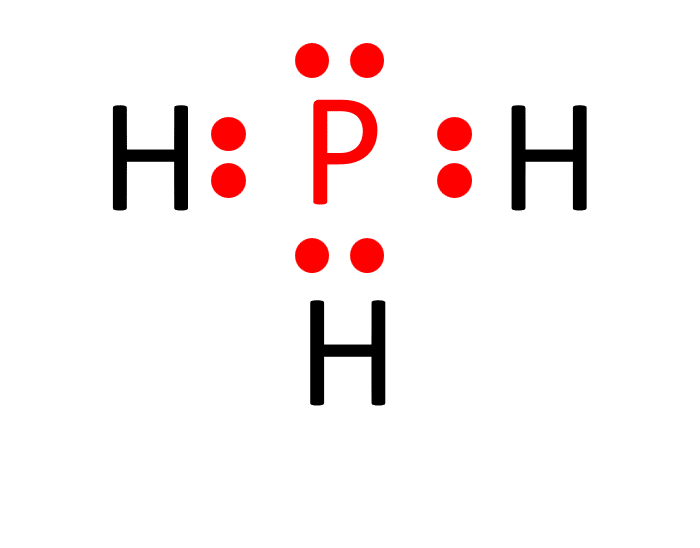
Molecular Shape of PH3:
The molecular shape of PH3 is trigonal pyramidal. In this configuration, the central phosphorus atom is bonded to three hydrogen atoms, and an unshared pair of electrons on phosphorus creates a pyramidal shape.
Bond Type in PH3:
In PH3, phosphorus forms single bonds with each of the three hydrogen atoms. These are sigma (σ) bonds, resulting from the overlap of atomic orbitals.
Properties of PH3:
Phosphine (PH3) is a colorless, flammable gas with a distinct, unpleasant odor often described as garlic-like. It is highly toxic and can be lethal when inhaled in significant quantities. PH3 is spontaneously flammable in the presence of air and is commonly used as a fumigant in agriculture.
Bond Angles in PH3:
The bond angles between the central phosphorus atom and the three hydrogen atoms in PH3 are approximately 93.5 degrees. This specific angle results from the repulsion of electron pairs around the central atom, leading to the characteristic trigonal pyramidal shape.
Hybridization in PH3:
Phosphorus in PH3 undergoes sp3 hybridization, involving the combination of one s orbital and three p orbitals of phosphorus to form four sp3 hybrid orbitals. These hybrid orbitals overlap with the s orbitals of hydrogen atoms, creating sigma bonds and maintaining the trigonal pyramidal molecular shape of PH3.
Conclusion:
In conclusion, understanding the Lewis structure, molecular shape, bond characteristics, and properties of PH3 provides valuable insights into its chemical behavior and significance in various applications. The choice of phosphorus as the central atom, bond angles, and bond lengths all contribute to the unique properties and reactivity of this compound, making it a crucial component in certain chemical processes and fumigation practices.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023