Hydrogen Cyanide (HCN) is a colorless, flammable, and poisonous liquid. The HCN Lewis structure comprises three different atoms: hydrogen, carbon, and nitrogen. It is a polar molecule with a bond angle of 180 degrees. HCN is used in electroplating, mining, and as a precursor for several compounds.
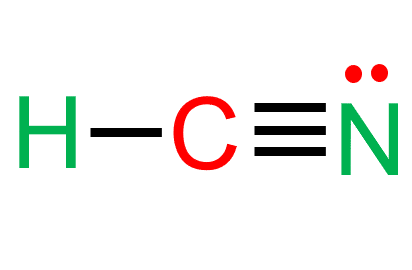
| Name of molecule | Hydrogen Cyanide (HCN) |
| Bond Angles | 180 degrees |
| Molecular Geometry of Hydrogen cyanide | Linear |
| Hybridization of Hydrogen cyanide | sp hybridization |
| No Valence Electrons in the molecule | 10 |
Table of Contents
Step-by-Step Construction of Lewis Structure
The following are the steps to constructing the Lewis Structure.
Step-1: Count the valence electrons of atoms
To draw a Lewis structure, we need to figure out the number of valence electrons in individual atoms as shown in the table below.
| Atom | Electronic Configuration | Valence Electrons (VEs) |
| 7N | 1S2 2S2 2P3 | 5 |
| 6C | 1S2 2S1 2P3 | 4 |
| 1H | 1S1 | 1 |
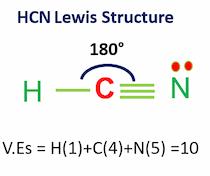
VEs= VEs in hydrogen + VEs in carbon + VEs in nitrogen
Valence electrons = 1+4+5 = 10
Step 2: Determine the central atom
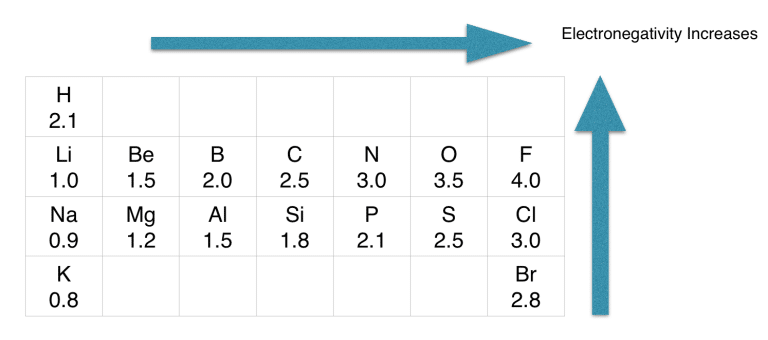
If we check the proper arrangement of C and N in the periodic table, we will find that the electronegativity values of C, N, and O are 2.5, 3.5, and 2.1.
As per the rule, the atom with the least electronegative value should be at the structure’s center.
Since hydrogen is the least electronegative, it can not take a central position.
And due to the difference in electronegativities between carbon and hydrogen, the vector represents the charge that will be drawn from hydrogen to carbon.
As carbon is a less electronegative atom than nitrogen in molecules, it will take the central position.
Place the hydrogen and nitrogen atoms on both terminal sides of the carbon.
Step 3: Place electron pairs between the atoms
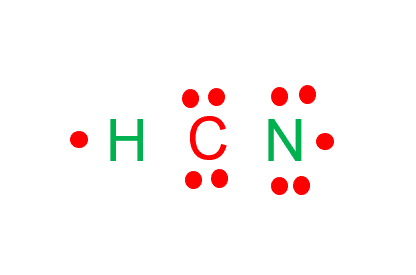
We need to distribute the 10 remaining valence electrons. Hydrogen will have one electron, carbon will have four electrons, and nitrogen will have five electrons.
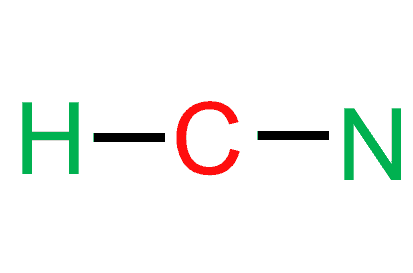
Step 4: Place the remaining electrons around the other atoms.
After making a single bond with hydrogen, C is left with only three valence electrons as it has shared one electron with hydrogen.
Therefore, carbon will share its remaining three electrons with nitrogen to complete its octet, resulting in the formation of a triple bond between carbon and nitrogen.
HCN Molecular Geometry
Hydrogen cyanide has linear molecular geometry with bond angles of 180 degrees.
As hydrogen and nitrogen tend to be far from each other, HCN forms a linear shape.
It is slightly polar as nitrogen tries to pull the electrons to itself due to its electronegative value.
Due to such differences, hydrogen will have slightly positive charges and nitrogen will have slightly negative charges as the vector goes from hydrogen to nitrogen.
Thus, nitrogen becomes a negative pole, and the hydrogen atom becomes a positive pole, making the molecular polar.
HCN Lewis Structure- Key Points
- colorless, extremely flammable substance
- The extremely poisonous liquid is produced on an industrial scale.
- density = 2.648 g/cm3
- The molar mass is 27.03 g/mol.
- In the HCN Lewis structure, there is a triple bond between carbon and nitrogen and a single bond between C and H.
- A solution of hydrogen cyanide in water is called hydrocyanic acid.

HCN Hybridization
There are two sigma bonds, C-H and C-N.
The bond between the C and the N in hydrogen cyanide is a triple bond.
The hybrid orbital is sp, due to the linear geometry of the molecule.
Hybridization of Carbon in Hydrogen cyanide
Carbon is triple-bonded to nitrogen, and so there are one sigma and two pi bonds.
As per the rule, the first bond between any two atoms is a sigma bond, and the second and third bonds are pi bonds)
This means two p orbitals are required to be left over after hybridization.
2 pi bonds = 2 leftover p orbitals.
As a result, one of carbon’s p orbitals is available to hybridize.
Hybridization of Nitrogen in Hydrogen cyanide
Nitrogen has two sp hybridized orbitals and two degenerate p orbitals.
One sp orbital participates in a sigma bond with the carbon atom’s sp hybridized orbital.
The other sp orbital houses a lone pair of electrons.
The two p orbitals each contain a single electron which partakes in pi bonds with 2 unpaired p orbital electrons of the Carbon atom.
So both carbon and nitrogen are sp hybridized.
Molar Mass of HCN
Molar mass of Hydrogen = 1.00794 g/mol
Carbon molar mass = 12.011 g/mol
Molar mass of N = 14.0067 g/mol
The molecular mass is 27.026 g/mol.
Uses of Hydrogen Cyanide
- Hydrogen cyanide is used in the preparation of acrylonitrile, which is used in the production of acrylic fibers, synthetic rubber, and plastics.
- Hydrogen cyanide and its compounds are used for many chemical processes, including fumigation, the case hardening of iron and steel, electroplating, and the concentration of ores.
- Hydrogen cyanide is an excellent solvent for many salts, but it is not widely used as a solvent because of its toxicity.
Is HCN Polar or Nonpolar?
HCN is a polar molecule.
The electronegativity difference between nitrogen (3.04) and hydrogen (2.2) makes it a polar molecule.
The electronegativity difference between atoms is directly proportional to the polarity of the molecule.
Carbon is at the center, surrounded by nitrogen and hydrogen atoms.
Carbon and hydrogen share electrons to form a covalent bond.
Whereas carbon and nitrogen form a triple bond to share three electrons.
This results in an unequal sharing of charge in the linear-shaped HCN molecule and a non-zero dipole moment.
Nitrogen gains a partial negative charge, whereas hydrogen gains a partial positive charge.
As a result, positive and negative poles are created across the molecule, and HCN becomes a polar molecule.
Important Links
Hydrogen Cyanide Effects
Hydrogen cyanide (HCN) poisoning can be fatal in a matter of minutes.
It can particularly affect those organ systems which are most sensitive to low oxygen levels, like the central nervous system (brain), the cardiovascular system (heart and blood vessels), and the pulmonary system (lungs).
Related Links
CO2 Lewis Structure and Molecular Geometry
SiO2 Lewis Structure
SO2 (Sulfur Dioxide) Lewis structure
N2O Lewis Structure| Laughing Gas
Co2 Polar or Nonpolar
Summary
To summarize everything in this article, the following are some important points:
- In the HCN Lewis structure, carbon forms one single bond with the hydrogen atom and a triple bond with the nitrogen atom.
- The bond angle is 180 degrees, and there are 10 valence electrons.
- HCN is a polar molecule with linear geometry.
- Exposure to hydrogen cyanide can be dangerous.
Frequently Asked Questions (FAQs)
Some of the frequently asked questions are given below
1. Why Hydrogen Cyanide is polar?
In hydrogen cyanide, carbon has an electronegativity of 2.5, hydrogen’s electronegativity is 2.1, and nitrogen has an electronegativity of 3. Any molecule that has a difference in electronegativities of any dipole moment is considered polar. Therefore, hydrogen cyanide is a polar molecule.
2. Explain Hydrogen Cyanide Lewis Structure in simple words
Hydrogen cyanide is a polar molecule with a triple bond between carbon and nitrogen. The structure is made up of three different atoms of hydrogen, carbon, and nitrogen. It is a polar molecule with bond angles of 180 degrees.
3. What is hydrocyanic acid?
A solution of hydrogen cyanide in water is called hydrocyanic acid.
4. What is cyanide poisoning?
Cyanide poisoning refers to the harmful effects of inhaling hydrogen cyanide or ingesting the salts of hydrogen cyanide, called cyanides.
5. Why Lewis structures are important?
Lewis structure is a simplified representation of valence shell electrons.
It depicts the arrangement of electrons around individual atoms in a molecule.
Electrons are shown as “dots” or as a line between two atoms when they are bonded.
6. How to draw the Lewis structure of oxygen?
In the O2 Lewis structure, there is a double bond between two oxygen atoms.
Oxygen is a diatomic nonpolar molecule with a bond angle of 180 degrees.
In its molecule, both oxygen atoms have the same electronegativity value, both atoms share equal ratios of bonded shared electrons, and the overall O2 molecule turns out to be nonpolar.
7. What is the dot structure of hydrogen sulfide?
On both sides of the central sulfur atom in the H2S Lewis structure, there are two hydrogen atoms.
The molecule bends due to the existence of two unbonded pairs of electrons.
The molecule is slightly polar because sulfur is more electronegative than hydrogen.
In the case of H2S, the vectorial sum of the bond dipole moments results in a non-zero total dipole moment. As a result, dipole-dipole interactions are observed in hydrogen sulfide.
8. What is CLF3 molecular geometry?
ClF3 has a T-shaped molecular geometry and a trigonal bipyramidal electron geometry. This molecule has two lone pairs and three bound pairs, according to the ClF3 Lewis structure. ClF3 is a polar compound.
Author
Umair Javed
Umair has been working at Whatsinsight since 2020 as a content writer.
He has a Master’s degree in Materials Science.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023