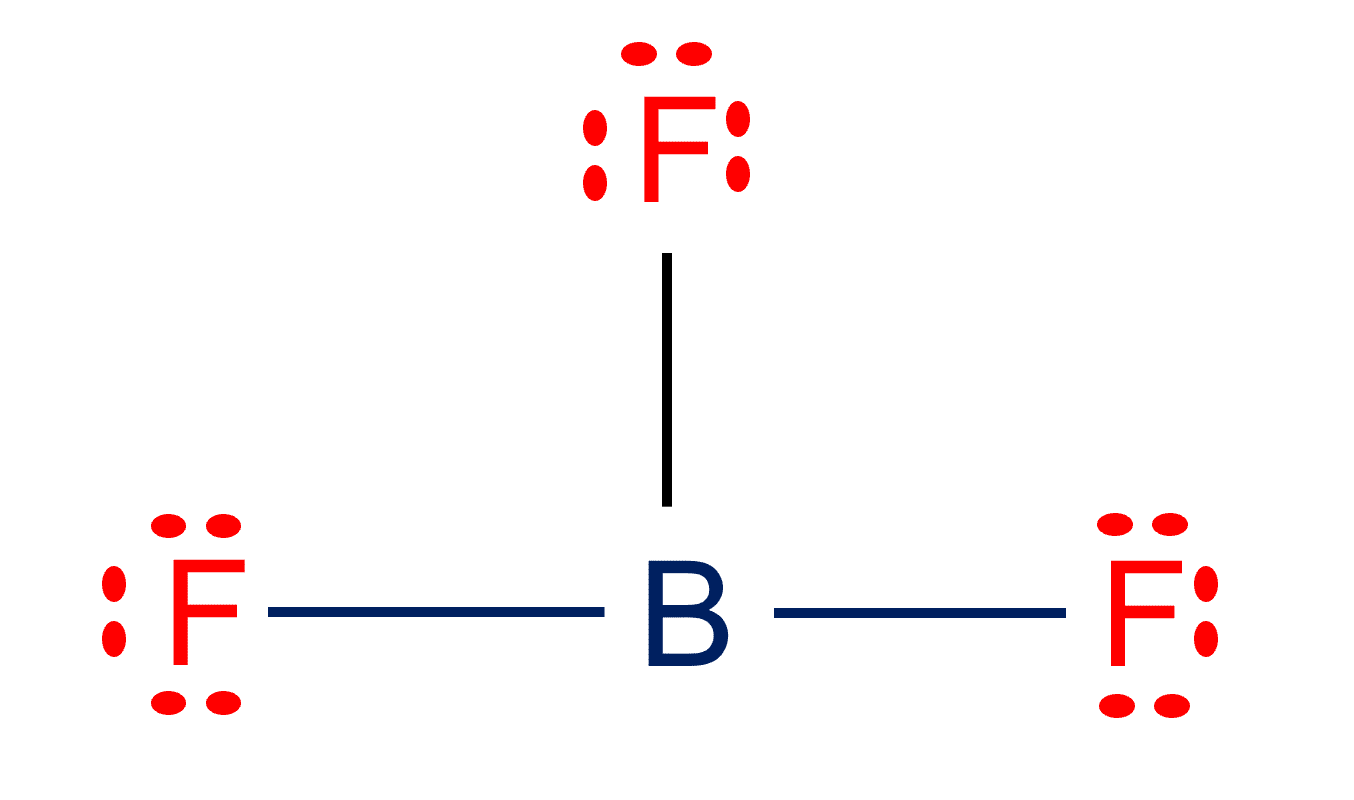
Boron trifluoride (BF3) is a non-polar inorganic chemical compound that is a colorless gas with a pungent smell. The BF3 molecule has 1 atom of Boron and 3 atoms of Fluorine. The highly symmetrical structure of BF3 allows the bond dipole moments to cancel, resulting in a molecular dipole moment of zero. BF3 molecular geometry is trigonal planer.
| Name of molecule | Boron trifluoride (BF3) |
| Bond Angles | 120° |
| BF3 Molecular Geometry | trigonal planar |
| No of Valence Electrons in the molecule | six valence electrons |
| Lone pairs of electrons | zero |
| Is BF3 Polar or Nonpolar? | Non-polar |
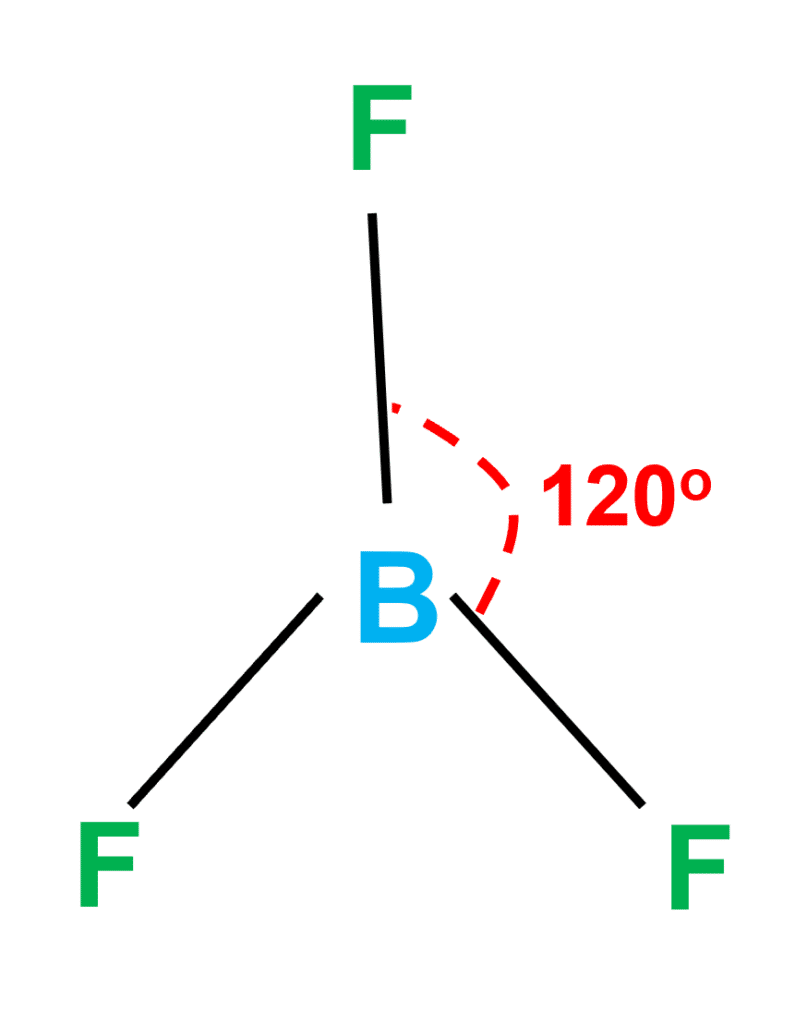
Table of Contents
Polarity of BF3
To understand the non-polar nature of BF3, we need to understand its structure.
The Boron Trifluoride molecule has one Boron atom and three Fluorine atoms. Boron has a valency of 3 and Fluorine has a valency of 7. The fluorine atom, on the other hand, contains three lone pairs of electrons, therefore the molecular structure is balanced and symmetric.
The table below gives details about the electronic configuration of constituent atoms and their valence electrons.
| Atom | Electronic Configuration | Valence Electrons (VEs) |
| Boron (B) | 1s22s22p1. | 3 |
| Fluorine (F) | 1s22s22p5 | 7 |
VEs in BF3 = VEs in 1 B atom + VEs in 3 F atoms
VEs= 1(3)+3(7) =24 electrons
Boron trifluoride contains just six valence electrons and is one of the rare second period covalent compounds that defies the octet rule. There are three bonded groups, hence there are no lone pairings. Six electrons imply three electron pairs and, as a result, a trigonal geometry.
Properties of Boron trifluoride
- Boron trifluoride is a colorless toxic gas.
- BF3 molar mass is 67.805 g mol-1.
- Boron trifluoride is soluble in water.
- It is also soluble in sulfuric acid and organic solvents such as benzene, carbon tetrachloride, sulfur dioxide, and chloroform.
- BF3 is used in the manufacture of adhesives and sealing chemicals, as well as lubricants and lubricant additives.
- It is also used as a catalyst in alkylation, esterification, and condensation reactions.
- Is BF3 Polar or Nonpolar: BF3 is nonpolar
What is Polarity?
Polarity is caused by the distribution of electrical charge across the atoms connected by the bond.
It is found, for example, that bonds between atoms of different elements are electrically inequivalent. For example, in hydrogen chloride, the H atom is slightly positively charged, whereas the Cl atom is slightly negatively charged.
The slight electrical charges on dissimilar atoms are called partial charges, and the presence of partial charges signifies the occurrence of a polar bond.
Examples of Polar molecules
Some examples of polar molecules are:
Examples of Non-Polar molecules
- Carbon dioxide
- Benzene
- Carbon tetrachloride
- Methane
- Ethylene
Non Polarity of CO2
Carbon dioxide is a nonpolar molecule that contains polar bonds.
CO2 is a linear molecule and the C=O are polar bonds.
The central carbon has a net positive charge, whereas the surrounding two oxygens have a net negative charge.
Due to the linearity of the carbon dioxide molecule, these two bond dipoles cancel each other out.
As a result, the molecule as a whole has no dipole.
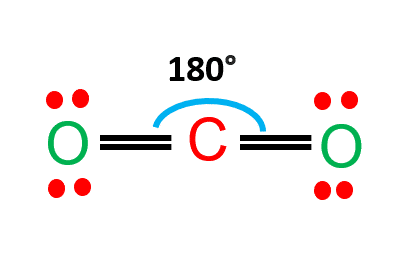
CO2 lewis structure shows CO2 molecules comprised of two oxygen atoms and one carbon atom that are covalently bound together. Two oxygen atoms are located on the sides of a carbon atom, where they share electrons and form bonds. Since the carbon atom is the least electronegative, it is present at the center of the molecule.
BF3 Molecular Geometry
BF3 molecular geometry is ‘Trigonal Planar’. According to Chemistry, a ‘Trigonal Planar’ model has three atoms around one atom in the center. It’s like peripheral atoms all on one plane, because all three of them are similar with 120° bond angles on each, forming an equilateral triangle.
Summary
Some of the summary points are listed below:
- The answer to the question “Is BF3 polar or nonpolar?” is that BF3 is non-polar.
- Boron trifluoride (BF3) is a non-polar inorganic chemical compound.
- There are a total of 24 valence electrons for the BrF3 Lewis structure
- BF3 molecular geometry is trigonal planer.
- Boron trifluoride is a colorless gas with a terrible and stifling odor.
Related Links
HCN Lewis Structure| Step By Step Construction
N2O Lewis Structure| Laughing Gas
SiO2 Lewis Structure
Co2 Polar or Nonpolar
H2S Lewis Structure & Molecular Geometry
SO2 Polar or Nonpolar
Frequently Asked Questions
Some of the frequent questions are given below. If you have any questions please feel free to post a comment.
1. What is the difference between ammonia and boron trifluoride?
Boron trifluoride is an inorganic compound with the chemical formula BF3, whereas ammonia is an inorganic compound with the chemical formula NH3. The primary distinction between ammonia and boron trifluoride is that ammonia is a polar molecule while boron trifluoride is a nonpolar molecule.
2. What is the structure of BF3?
The structure formed in the plane shows that BF3’s molecular geometry is trigonal planar (central atoms are surrounded by three-terminal atoms). This geometry forms an equilateral triangle with a 120-degree angle on each side.
3. Is BF3 Polar or Nonpolar?
BF3 is a non-polar compound. In BF3, the central boron atom has sp2 hybridized orbitals, resulting in an unfilled p orbital on the Bron atom and trigonal planar molecular geometry. Because the Boron-Fluorine bonds are all 120 degrees apart, any net dipole in that plane is canceled out. Even if each B-F bond is polar, the net dipole moment is zero because adding the bond vectors cancels everything out.
4. Is Hydrogen sulfide (H2S) polar or nonpolar?
Hydrogen sulfide (H2S) is a colorless gas with a strong “rotten egg” odor.
The difference in electronegativity values of the Hydrogen (2.2) and Sulfur (2.58) atoms makes H2S a slightly polar molecule.
Furthermore, the existence of two lone pairs on opposing sides of the two Hydrogen atoms makes the molecule more polar and results in the bending shape geometrical structure of H2S. Check the full article “Is H2S polar or nonpolar?”.
5. Is SiCl4 polar or nonpolar?
SiCl4 (silicon tetrachloride) is a nonpolar molecule. Because the four chemical bonds between silicon and chlorine are uniformly distributed, SiCl4 is non-polar. A polar covalent bond is a type of covalent link that is intermediate between pure covalent bonds and ionic bonds. When the difference in electronegativity between the anion and the cation is between 0.4 and 1.7, such bonds occur.
Check the full article “Is SiCl4 polar or nonpolar?”.
6. NH3 is a polar or nonpolar molecule?
Ammonia (NH3) is a polar molecule. Since the molecule contains three N-H bond dipoles that do not cancel out. In each bond, nitrogen is more electronegative than hydrogen. This unequal distribution of charges among both nitrogen and hydrogen atoms makes NH3 a polar molecule.
7. NH3 oxidation number?
The NH3 oxidation number is the sum of individual oxidation numbers of the atoms nitrogen (oxidation number = -3) and hydrogen (oxidation number = 1) is zero.
8. What is nitrogen trifluoride gas?
Nitrogen trifluoride (NF3) is an odorless, colorless gas. When inhaled, it is exceedingly poisonous. Containers may burst violently if exposed to fire or heat for a lengthy period of time.
9. What is perchloric acid used for?
Perchloric acid (HCLO4) is used to separate potassium from sodium in many laboratory tests and industrial processes. It is also used as an oxidizing agent, especially in the determination of chromium in steel, ferrochrome, chromite, and leather.
10. Is SO2 an ionic or covalent compound?
When the electronegativity difference between two elements is between 0 (two of the same element bonding together) and 1.69, a covalent bond is created (almost ionic bonding). The oxygen element has an electronegativity of 3.5 in an SO2 molecule while the sulfur element has an electronegativity of 2.6; the electronegativity difference between the two elements is 0.9. As a result, SO2 is a covalent molecule that is also somewhat polar.
Conclusion
This article answers the question “Is BF3 Polar or Nonpolar” and BF3 molecular geometry.
We hope after reading the article, you are able to understand the non-polar nature of BF3 and its molecular structure of BF3. If you have any questions regarding this topic, feel free to post a comment.
More Links
Is HCl Polar or Nonpolar?
CH4 Lewis Structure & Molecular Geometry
Is HCN Polar or Nonpolar?
CO2 Lewis Structure and Molecular Geometry
Is O2 Polar or Nonpolar?| Easy Explanation
Disclaimer
Whatsinsight.org‘s blog and everything published on it are provided solely as an information resource. The blog, its authors, and affiliates accept no responsibility for any accident, injury, or damage caused by following the information on this website, in part or in whole. We do not recommend using any chemical without first consulting the manufacturer’s Material Safety Data Sheet and following the safety advice and precautions on the product label.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023