Ammonia (NH3) is a colorless, pungent gas. With three hydrogen atoms and an unshared pair of electrons connected to the nitrogen atom, the NH3 molecular geometry is of a trigonal pyramidal shape.
So, is NH3 an acid or base? NH3 has a basic character because when NH3 dissolves in an aqueous solution, NH3 absorbs the H+ ion from a water molecule ion and generates hydroxide ions (OH–). However, depending on who it reacts with, NH3 can also behave as an acid, but ammonia is generally a weak basic.
| Name of molecule | Ammonia (NH3) |
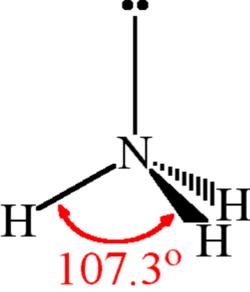
| Bond Angles | 107.3 degrees |
| Molecular Geometry of NH3 | Pyramidal planar |
| Hybridization of NH3 | SP3 hybridization |
| Electronic configuration | 1S22S22P3. |
| NH3 oxidation number | zero |
| NH3 acid or base? | Weak base |
| Conjugate acid | NH4+ |
| Conjugate base | NH2– |
| Basicity (pKb) | 4.75 |
| Solubility in water | miscible |
Table of Contents
NH3 Molecular Geometry
Ammonia (NH3) has a trigonal, pyramidal, or distorted tetrahedral molecular shape.
It is due to the presence of a single non-bonding lone pair of electrons on the nitrogen atom, which acts as a repulsive force on the bonding orbitals.
In the center of the NH3 molecular geometry, three hydrogen atoms are connected to a nitrogen atom.
Because nitrogen has 5 electrons in its valence shell, it must interact with 3 hydrogen atoms to satisfy the octet rule and form ammonia, a stable molecule.
The unbonded electrons are called lone pairs of electrons.
Is NH3 Acid or Base?
Ammonia is a relatively weak base. It does not contain hydroxide ions on its own, but it creates ammonium and hydroxide ions when reacting with water.
A weak base does not completely break down into its component ions when dissolved in liquids.
Lewis Structure of NH3
NH3 Lewis structure shows that ammonia contains three hydrogen atoms and an unshared pair of electrons attached to the nitrogen atom.
In a noble gas structure (complete shell), each hydrogen atom loses one electron, whereas nitrogen lacks three electrons in a whole outer shell (of 8).
Ammonia is made up of three hydrogen atoms and one nitrogen atom.
The NH3 molecule is held together by the strong N–H nitrogen–hydrogen single covalent bonds by sharing electrons. The H–N–H bond angle is 107o.
The table below shows the number of valence electrons in ammonia.
| Atom | Electronic Configuration | Valence Electrons (VEs) |
| 7N | 1s22s22p3 | 5 |
| 1H | 1s1 | 1 |
VEs = VEs in three hydrogen atoms + VE in one nitrogen atom
Valence electrons = 3(1)+5 =8
What is Ammonia?
Ammonia is a nitrogen-hydrogen compound that is also known as hydrogen nitride.
In the main reformer, natural gas, specifically Methane, is reacted with steam to make hydrogen.
The air is added to the gas stream. In the secondary reformer.
In the converter, leftover hydrogen and nitrogen from the air mix in a 3:1 ratio to produce ammonia.
Ammonia- Key Points
- Ammonia is a colorless gas.
- It is easily liquefied due to the strong hydrogen bonding between molecules.
- Pungent odor.
- The ammonia molecule exhibits a trigonal pyramidal shape.
- The melting point is -77.73°C.
- The boiling point is -33.34 °C.
- The density is 0.73 kg/m3.
- Molar mass of ammonia = 17.03052 g/mol.
- classified as an extremely hazardous substance.
- It is used as a raw material to make a variety of commercially significant nitrogen compounds.
Molar Mass of Ammonia
The molecular mass of nitrogen is 14.0067 g/mol.
Hydrogen Molar Mass = 1.00794 g/mol.
Molar mass of Ammonia (NH3)= 14.0067 g/mol + (3× 1.00794) g/mol = 17.03052 g/mol
= 17.03052 g/mol
Is NH3 Polar?
NH3 is polar because it has three N-H bond dipoles that do not cancel out. In each bond, nitrogen is more electronegative than hydrogen. The uneven distribution of charges among nitrogen and hydrogen atoms causes polarity.
The N–H bond is polar because of the electronegativity difference between N (3.04) and H (2.2). The three N-H bonds produce three dipole moments in one direction due to the variation in their electronegativities.
The three dipoles pointing in the same direction provide a net dipole moment, which creates polarity.
Furthermore, the lone pair on the nitrogen atom exerts an outward strain on the bond, causing the structure of NH3 to become asymmetrical.
Summary
- Ammonia is a relatively weak base. It does not contain hydroxide ions on its own, but when treated with water, it produces ammonium and hydroxide ions.
- NH3 molecular geometry is of trigonal pyramidal shape.
- Many industries use the Haber process to produce ammonia on an industrial scale.
- Some well-known uses of ammonia are dye-making, preparing medicines, and production of fertilizers.
- NH3 Lewis structure shows that there are three N-H bonds and one lone pair
Related Topics
CO2 Lewis Structure and Molecular Geometry
HCN Lewis Structure| Step By Step Construction
SiO2 Lewis Structure
N2O Lewis Structure| Laughing Gas
Charge of Ammonia (NH3)| Simple Steps
HCN (Hydrogen Cyanide) Hybridization
Frequently Asked Questions (FAQs)
Below are some of the frequently asked questions
1. Is NH3 fetal?
Exposure to 300 ppm is dangerous to life and health (IDLH) and can be fatal within a few breaths. Ammonia is corrosive to the skin, eyes, and lungs.
2. What is the difference between ammonia and ammonium?
NH3 (ammonia) is a toxic gas.
Ammonium is a nontoxic salt. It is the ionized form of ammonia.
Its ions are produced during ammonification.
3. What pH is ammonia?
Ammonia consists of one negatively charged nitrogen ion and three positively charged hydrogen ions. The pH of standard ammonia is about 11.
Bottom Line
This article explains the Ammonia molecular geometry, Lewis structure, and arrangement of atoms in NH3. Please feel to comment if you have any questions.
Author
Umair Javed
Umair has been working at Whatsinsight since 2020 as a content writer.
More Interesting Topics
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023