Ammonia (NH3) is a colorless, pungent gas. With three hydrogen atoms and an unshared pair of electrons connected to the nitrogen atom, NH3 molecular geometry is of Trigonal Pyramidal shape.
| Name of molecule | Ammonia (NH3) |
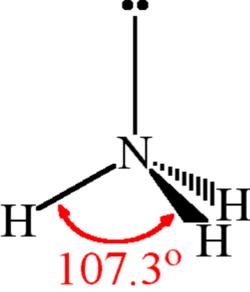
| Bond Angles | 107.3 degrees |
| Molecular Geometry of NH3 | Pyramidal planar |
| Hybridization of NH3 | SP3 hybridization |
| Electronic configuration | 1S22S22P3. |
| NH3 oxidation number | zero |
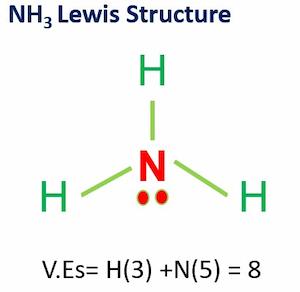
Table of Contents
Molecular Geometry of NH3
Ammonia (NH3) has a trigonal pyramidal or distorted tetrahedral molecular shape. It is due to the presence of a single non-bonding lone pair of electrons on the nitrogen atom, which acts as a repulsive force on the bonding orbitals.
In NH3 molecular geometry, three hydrogen atoms are bonded to a nitrogen atom in the middle.
Because nitrogen has 5 electrons in its valence shell, it must interact with 3 hydrogen atoms to satisfy the octet rule and produce ammonia, a stable molecule.
The unbonded electrons are called lone pairs of electrons.
Lewis Structure of NH3
NH3 Lewis structure shows that ammonia contains three hydrogen atoms and an unshared pair of electrons attached to the nitrogen atom.
Each hydrogen atom is missing one electron from a noble gas structure (complete shell), while nitrogen is missing three electrons from a full outer shell (of 8).
Three hydrogen atoms and one nitrogen atom combine to form ammonia so that the hydrogen atoms are electronically like helium and the nitrogen atom becomes like neon.
The NH3 molecule is held together by the strong N–H nitrogen–hydrogen single covalent bonds by sharing electrons. The H–N–H bond angle is 107o.
The table below shows the number of valence electrons in Ammonia
| Atom | Electronic Configuration | Valence Electrons (VEs) |
| 7N | 1s22s22p3 | 5 |
| 1H | 1s1 | 1 |
VEs = VEs in three Hydrogen atoms + VE in 1 Nitrogen atom
Valence electrons= 3(1)+5 =8
Electronic Configuration of Nitrogen in Ammonia
- Nitrogen in its ground state has configuration 1S22S22P3.
- During hybridization, one s orbital and 3p orbitals hybridize to form four hybrid orbitals of equal energy levels, making SP3 hybridization.
- Three half-filled SP3 orbitals of Nitrogen form a bond with three Hydrogen atoms.
- The fourth full-filled hybridize orbital holds the lone pair of electrons.
- The trigonal structure of ammonia is due to repulsive lone pair-bond pair attraction.
- As a result, the bond angle in the Ammonia is 107o which is less than the standard 109.5o.
- Strong intermolecular hydrogen bonding makes it highly associated.
- It is a polar molecule. The compound ammonia is formed when three hydrogen atoms interact with one nitrogen atom.
What is meant by Molecular Geometry?
The three-dimensional shape or arrangement of atoms in a molecule is known as molecular geometry or molecular structure. Understanding a compound’s molecular structure can aid in determining the compound’s polarity, reactivity, phase of matter, color, magnetism, and biological activity.
What is Ammonia?
Ammonia, also known as hydrogen nitride, is a nitrogen-hydrogen chemical.
It is produced by a simple process.
In the main reformer, natural gas, specifically Methane, is reacted with steam to make hydrogen.
The air is introduced to the gas stream in the secondary reformer.
The residual hydrogen and nitrogen from the air combine in the converter in a 3:1 ratio to make ammonia.
Ammonia- Key Points
- Ammonia is a colorless gas.
- Easily liquefied due to the strong hydrogen bonding between molecules.
- Pungent odor.
- The ammonia molecule exhibits a trigonal pyramidal shape.
- The melting point is -77.73 °C.
- The boiling point is -33.34 °C.
- Density is 0.73 kg/m³.
- Molar mass of Ammonia = 17.03052 g/mol.
- Classified as an extremely hazardous substance.
- It is used as a raw material to make a variety of commercially significant nitrogen compounds
Molar Mass of Ammonia
Molar mass of Nitrogen = 14.0067 g/mol.
Hydrogen Molar mass = 1.00794 g/mol.
Molar mass of Ammonia (NH3)= 14.0067 g/mol + (3× 1.00794) g/mol = =17.03052 g/mol
=17.03052 g/mol
Is Ammonia is a strong base?
Ammonia is a rather weak base. It doesn’t contain hydroxide ions on its own, but when it combines with water, it produces ammonium and hydroxide ions.
When dissolved in liquids, a weak base does not entirely break down into its constituent ions.
Is NH3 Polar?
NH3 is polar because it has three N-H bond dipoles that do not cancel out. In each bond, nitrogen is more electronegative than hydrogen. The uneven distribution of charges among nitrogen and hydrogen atoms causes polarity.
The N–H bond is polar because of the electronegativity difference between N (3.04) and H (2.2). The three N-H bonds produce three dipole moments in one direction due to the variation in their electronegativities.
The three dipoles in one direction generate a net dipole moment which causes polarity.
In addition, the lone pair on the nitrogen atom exerts an outward force on the bond due to which the shape of NH3 becomes unsymmetrical.

Uses of Ammonia
- Ammonia is used in the manufacture of explosives.
- Dye making.
- Preparing medicines.
- Used in the production of fertilizers.
- Ammonia is also used in household cleaning products.
Summary
- NH3 molecular geometry is of trigonal pyramidal shape.
- Many industries use the Haber process to produce ammonia on an industrial scale.
- Some well-known uses of ammonia are dye-making, preparing medicines, and production of fertilizers.
- NH3 Lewis structure shows that there are three N-H bonds and one lone pair
Related Topics
CO2 Lewis Structure and Molecular Geometry
HCN Lewis Structure| Step By Step Construction
SiO2 Lewis Structure
N2O Lewis Structure| Laughing Gas
Charge of Ammonia (NH3)| Simple Steps
Frequently Asked Questions (FAQs)
Below are some of the frequently asked questions
1. What is the oxidation number of NH3?
Ammonia is made up of one nitrogen atom and three hydrogen atoms (NH3). The NH3 oxidation number is zero because the sum of the individual oxidation values of the atoms nitrogen (oxidation number = -3) and hydrogen (oxidation number = 1) is zero.
Exposure to 300 ppm is dangerous to life and health (IDLH) and can be fatal within a few breaths. Ammonia is corrosive to the skin, eyes, and lungs.
NH3 (ammonia) is a toxic gas.
Ammonium is a nontoxic salt. It is the ionized form of ammonia.
Its ions produce during ammonification.
Ammonia consists of one negatively charged nitrogen ion and three positively charged hydrogen ions. The pH of standard ammonia is about 11.
Bottom Line
This article explains the Ammonia molecular geometry, Lewis structure and arrangement of atoms in NH3. Please feel to comment if you have any questions.
Author
Ms. Sana Javed
Sana has been working at Whatsinsight since 2020 as a content writer.
She has a Mphil degree in Pharmaceutics.
More Interesting Topics
CH4 Lewis Structure & Molecular Geometry
CO Lewis Structure & Molecular Geometry
O2 Lewis Structure & Molecular Geometry
SO2 Lewis Structure| 4 Simple Steps
Oxygen valence electrons
N2 Lewis Structure| Hybridization & Molecular Geometry
Chemical Reactions| Examples in Daily Life
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023