Ammonification is a process of converting naturally existing nitrogen to ammonia (NH3).
In this process, NH2 groups are converted into ammonia or its ionic form, ammonium (NH4+), as an end product.
Table of Contents
Ammonification Definition
Ammonification is defined as the process of converting natural nitrogen compounds into ammonia.
Ammonification in Simple Terms
- Ammonification is a natural process.
- It converts organic nitrogen into ammonia.
- This process is carried out by certain types of bacteria and fungi in soil or water.
- Ammonification occurs during the decomposition of dead plant and animal matter, as well as during the breakdown of organic nitrogen-containing compounds like fertilizer or sewage.
- The ammonia produced during ammonification is an important source of nitrogen for plants and other organisms.
- The ammonia can be absorbed by roots and used for growth.
- Ammonification helps to recycle nitrogen and other nutrients back into the ecosystem.
- This ensures that they remain available for future generations of living things.
Real-Life Examples
| Examples of Ammonificaction | Description |
| Decomposition of animal waste | Bacteria and fungi in the soil break down the nitrogen compounds in animal waste, releasing ammonia as a byproduct. |
| Decay of dead plant matter | Decomposers break down the organic nitrogen in dead plants, releasing ammonia into the soil. |
| Composting | Bacteria and fungi break down the organic nitrogen in composting materials, releasing ammonia as a byproduct. |
| Sewage treatment | Bacteria are used to convert organic nitrogen compounds in wastewater into ammonia, which can then be removed from the water. |
| Agricultural practices | Organic fertilizers like animal manure are broken down by soil microorganisms, releasing ammonia for plant uptake. |
Short Questions
Q: What is ammonification?
A: Ammonification is the process by which organic nitrogen is converted into ammonia by bacteria and fungi in the soil or water.
Q: What is the source of ammonia during ammonification?
A: The source of ammonia during ammonification is organic nitrogen, which is found in dead plant and animal matter, as well as in organic nitrogen-containing compounds like fertilizer or sewage.
Q: How does ammonification contribute to the nitrogen cycle?
A: Ammonification is an important part of the nitrogen cycle, as it helps to recycle nitrogen and other nutrients back into the ecosystem. The ammonia produced during ammonification can be used by plants and other organisms, and eventually, the nitrogen is returned to the soil or water through the process of denitrification.
Q: What types of organisms are involved in ammonification?
A: Bacteria and fungi are the primary types of organisms involved in ammonification.
Q: What is the role of ammonification in agriculture?
A: Ammonification is an important process in agriculture because it helps to break down organic nitrogen-containing compounds like fertilizer or manure, releasing ammonia that can be used by plants for growth.
Q: How does the process of ammonification contribute to the environment?
A: Ammonification is a natural process that helps to recycle nutrients and maintain a healthy ecosystem. It contributes to the availability of nitrogen for plants and other organisms and helps to prevent pollution by removing organic nitrogen compounds from water and soil.
Summary
- In Ammonification, microscopic organisms like bacteria or other types of decomposing organisms, break down nitrogen-containing chemicals from dead organic matter, into simple substances like ammonia.
- When an organism excretes waste or dies, the nitrogen in its tissues is in the form of organic nitrogen (e.g. amino acids, DNA).
- The Ammonification process converts this naturally existing nitrogen into inorganic ammonia or ammonia ions.
- During this process of oxidation of organic nitrogen to NH3, Bacteria and related microorganisms extract useful energy.
- The products of ammonification are ammonia and ammonium ions.
- Various fungi and prokaryotes then decompose the tissue and release inorganic nitrogen back into the ecosystem.
- In marine ecology, ammonification is also referred to as ammonium regeneration and ammonium recycling.
- The bacteria involved in the ammonification process are known as ammonifying bacteria.
- Examples of ammonifying bacteria are Bacillus, proteus, clostridium, Pseudomonas, and streptomyces.
Frequently Asked Questions
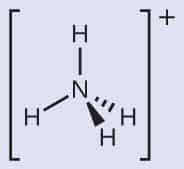
1. What is the oxidation number of NH3?
Ammonia is made up of one nitrogen atom and three hydrogen atoms (NH3). The NH3 oxidation number is zero because the sum of the individual oxidation values of the atoms nitrogen (oxidation number = -3) and hydrogen (oxidation number = 1) is zero.
2. What is the Lewis Structure of NH3?
NH3 Lewis structure shows that ammonia contains three hydrogen atoms and an unshared pair of electrons attached to the nitrogen atom.
Each hydrogen atom lacks one electron in a noble gas structure (complete shell), but nitrogen lacks three electrons in a full outer shell (of 8).
Three hydrogen atoms and one nitrogen atom combine to make ammonia, which has the same electrical properties as helium and the same physical properties as neon.
The NH3 molecule is held together by the strong N–H nitrogen–hydrogen single covalent bonds by sharing electrons. The H–N–H bond angle is 107o.
The table below shows the number of valence electrons in Ammonia
| Atom | Electronic Configuration | Valence Electrons (VEs) |
| 7N | 1s22s22p3 | 5 |
| 1H | 1s1 | 1 |
VEs = VEs in three Hydrogen atoms + VE in 1 Nitrogen atom
Valence electrons= 3(1)+5 =8
Related Topics
nh3 polar or nonpolar
How cold is Liquid Nitrogen?
NH3 Molecular Geometry| Trigonal Pyramidal
Charge of Ammonia (NH3)| Simple Steps
Ammonium Sulfide Formula
Valence Electrons in Nitrogen
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023