The molar mass of methanol is 32.04 g/mol. The methanol formula is CH3OH and it is also known as methyl alcohol, wood alcohol, or wood spirit. Keep reading to learn more about the structure, uses, and properties of methanol.
| Compound Name | Methanol |
| Other names | Methyl alcohol, wood alcohol, or wood spirit |
| Geometry | Tetrahedral geometry |
| Molar mass | 32.04 g/mol |
| Density | 0.792 g/cm3 |
| Melting point | −97.6 °C (−143.7 °F; 175.6 K) |
Table of Contents
Methanol Molecular Shape
The existence of lone pair-lone pair repulsions, lone pair-bond pair repulsions, and bond pair-bond pair repulsions in a molecule causes the varied forms in a given type of geometry.
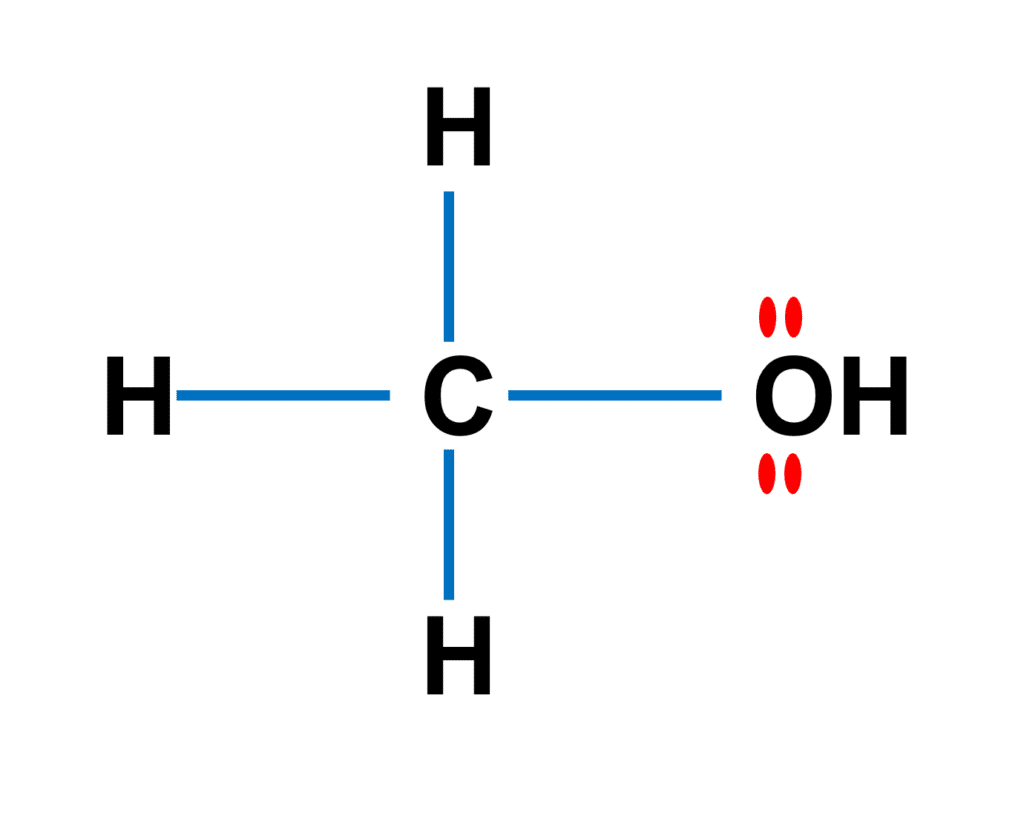
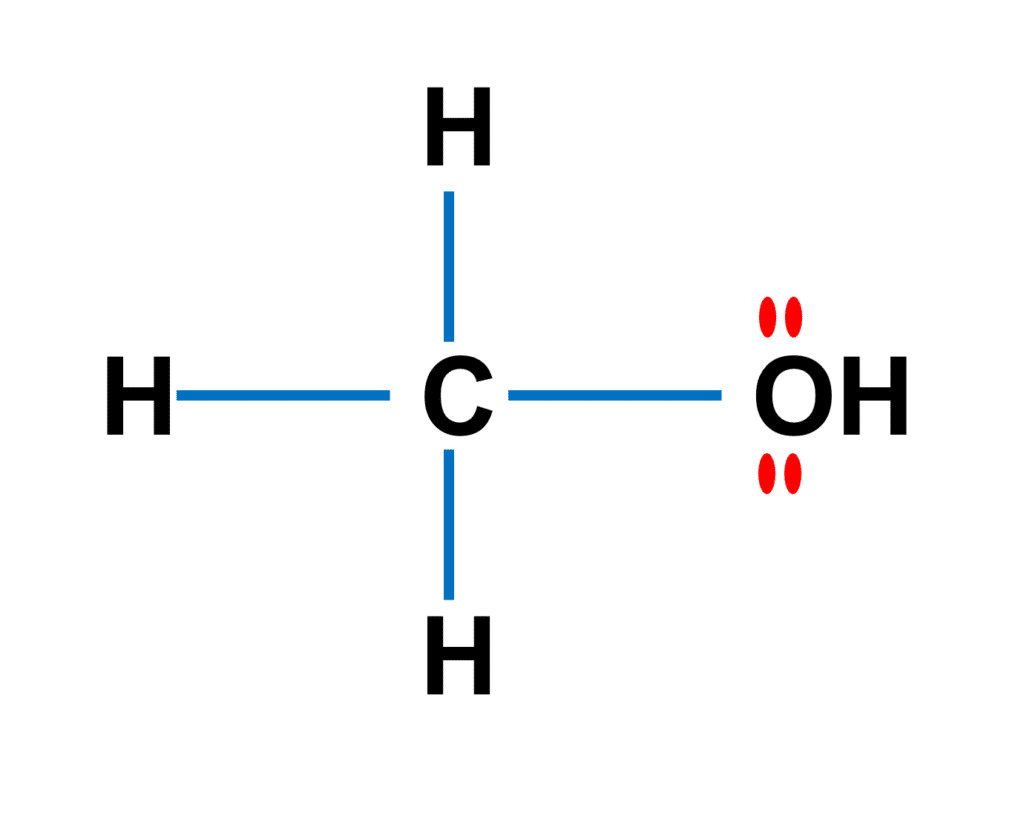
As shown in the figure above, Methanol (CH3OH) is the most basic alcohol, as it contains only one carbon atom. According to the Lewis structure, methanol has one O-H bond, three C-H bonds, and one C-O bond. On the oxygen atom, there are two lone pairs. The molecule as a whole has 14 electrons in valence shells as lone pairs and connections.
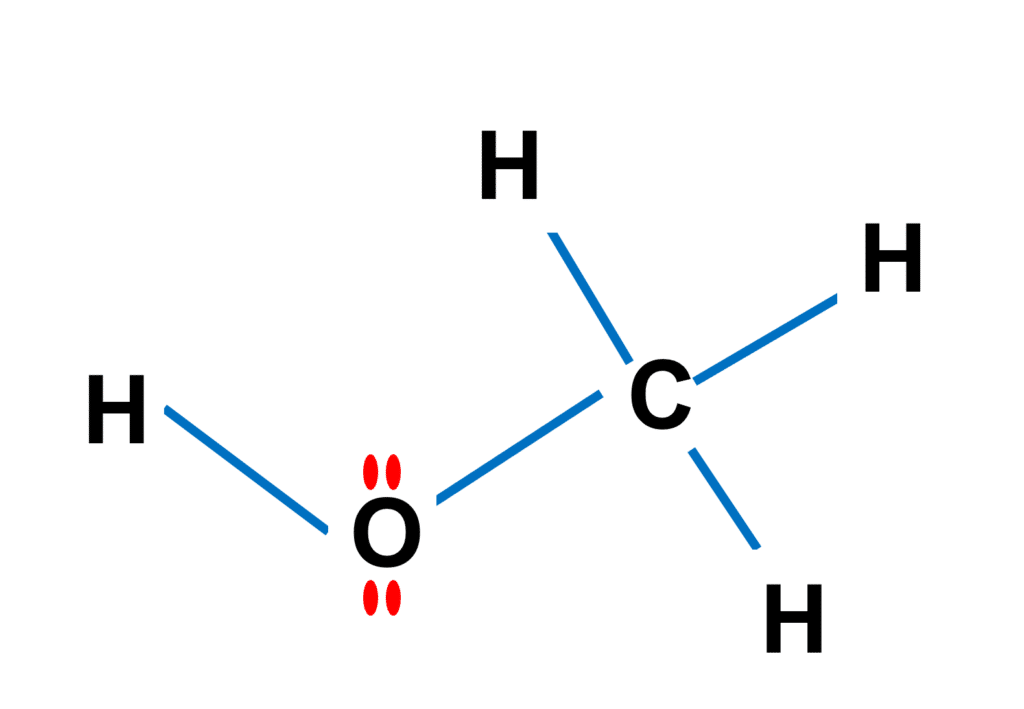
The central carbon atom in methanol possesses four sigma bonds and no lone pairs, implying that the molecule has a tetrahedral shape with equal bond angles of 109.5 degrees.
Because the oxygen atom has two sigma bonds and two lone pairs, methanol has a bent shape with a bond angle of 104.5 degrees with respect to the oxygen atom.
Molar Mass-CH3OH
(Atomic masses : C = 12u; H = 1u; O = 16u).
Molar mass of carbon atom = 12.01g/mol
The molar mass of Hydrogen atoms = 1.00794 g/mol.
Oxygen atom molar mass = 16.00 g/mol
The molar mass of CH3OH= molar mass of 1C + molar mass of 4H + molar mass of O
The molar mass of CH3OH=12.01 g/mol+ 4(1.00794 g/mol) + 16.00 g/mol = 32.04 g/mol.
Molar Mass of Methanol-Key Points
- colorless liquid.
- sweet and pungent order.
- forms explosive mixtures with air and burns with a nonluminous flame.
- completely miscible in water
- It easily evaporates at room temperature due to its low boiling point, and its fumes are always present. The fumes and liquid of methyl alcohol are exceedingly flammable.
Methanol Polarity
Methanol has three C-H bonds, one C-O bond, and one O-H bond. We need to calculate the electronegativity difference between a C-H, a C-O, and an O-H bond.
The electronegativity of C is 2.55, O is 3.44, and H is 2.2.
| Bond | Electronegativity difference | Bond type |
| C-H bond | 2.55 – 2.2 = 0.35 | Nonpolar |
| C-O bond | 3.44 – 2.55 = 0.89 | Polar |
| O-H bond | 3.44 – 2.2 = 1.24 | Polar. |
The electron density of the bond is shifted more towards oxygen because oxygen is more electronegative than carbon or hydrogen (as shown by electronegativity values), and the two dipole moments do not cancel each other out.
What is Methanol?
Methanol is the primary alcohol that is the simplest aliphatic alcohol, comprising only a methyl and an alcohol group.
It is a toxic toxin that may enter the body via the eyes, skin, lungs, and digestive system. It is utilized in a variety of sectors. It is used in the production of plastics, polyesters, and other compounds, as well as solvents and deicers.
Overexposure can be fatal. Workers may be injured if they are exposed to methyl alcohol. The amount of injury is determined by the dose, duration, and task performed.

Methanol Uses
- Methanol is used in the production of chemicals such as acetic acid and formaldehyde, which are then utilized in goods such as adhesives, foams, plywood subfloors, solvents, and windshield washer fluid.
Related Links
CO2 Lewis Structure and Molecular Geometry
HCN Lewis Structure| Step By Step Construction
SO2 (Sulfur Dioxide) Lewis structure
N2O Lewis Structure| Laughing Gas
Summary
To summarise, CH3OH is a polar and neutral molecule with tetrahedral geometry and bent and tetrahedral forms with respect to the oxygen and carbon atoms.
Frequently Asked Questions
1. How many electrons does iron have?
Iron contains eight electrons in its valence shell. Because iron is a transition metal, electrons in its d subshells can be used as valence electrons. Valence electrons are electrons that exist outside of a noble-gas core in a transition metal. Iron has the electrical configuration 1s22s22p63s23p64s23d6.
2. How many electrons does oxygen have?
A single oxygen atom has eight protons, eight electrons, and eight neutrons.
Oxygen is a stable isotope of oxygen with a nucleus of 8 neutrons and 8 protons. Its mass is 15.99491461956 u. Check full topic “How many electrons does oxygen have?”.
3. How many valence electrons does sodium have?
Sodium, like all other alkali metals in group 1, has a single valence electron. Valence electrons are the outermost electrons from the nucleus and are involved in bonding.
4. What is the valence of magnesium?
Magnesium has the atomic number 12. It has an electrical configuration of 2,8,2. As a result, it contains two valence electrons.
5. How many valence electrons does chlorine have?
Chlorine has the atomic number 17. It has seven valence electrons.
Check the related article “Is chlorine a metal?”.
6. Molar mass of methanol?
The molar mass of methanol is 32.04 g/mol. The methanol formula is CH3OH and it is also known as methyl alcohol, wood alcohol, or wood spirit.
7. Is liquid nitrogen dry ice?
The difference between dry ice and liquid nitrogen is that dry ice is a solid form of carbon dioxide, whereas liquid nitrogen is elemental nitrogen in liquid form.
Check the full article “How cold is liquid nitrogen?”.
Author
Umair Javed
Umair has been working at Whatsinsight since 2020 as a content writer.
He has a Masters degree in Materials Science.
More Links
Liquid Definition| Chemistry
London Dispersion Force| Definition and Examples
Ionic Radius| Definition, and Trends
Enthalpy Definition| Chemistry
Absolute Alcohol| Definition, and Formula
Disclaimer
Whatsinsight.org‘s blog and everything published on it are provided solely as an information resource. The blog, its authors, and affiliates accept no responsibility for any accident, injury, or damage caused by following the information on this website, in part or in whole. We do not recommend using any chemical without first consulting the manufacturer’s Material Safety Data Sheet and following the safety advice and precautions on the product label.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023