The charge of ammonia is zero.
Ammonia (NH3) comprises one nitrogen atom and three hydrogen atoms. The charge number of an atom reflects the fact that the valence shell of an atom will typically contain all of its electrons, regardless of whether the atom gains or loses electrons to accomplish this. Keep reading to know how the charge of ammonia is zero?.
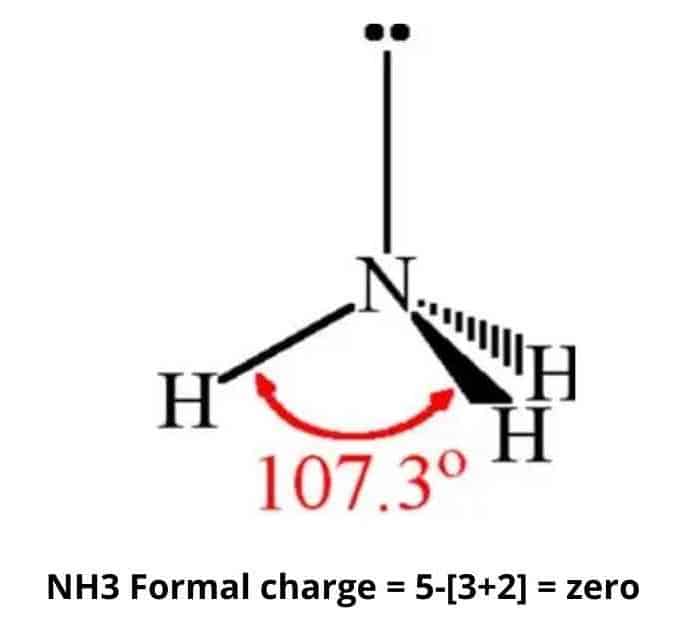
Table of Contents
Charge of Ammonia (NH3)
Formal Charge = [the number of the valence electrons in the atom] – [(the number of non-bonded electrons) + (the number of bonds)].
As per the NH3 Lewis structure, the nitrogen atom in ammonia has one lone pair and three bonds with hydrogen atoms. In addition, the neutral hydrogen atom has one valence electron. Each hydrogen atom in the molecule has no nonbonding electrons and one bond.
Formal Charge of Nitrogen = (5 valence e-) − (2 lone pair e-) − (1/2 x 6 bond pair e-) = 0
Formal Charge of Hydrogen = (1 valence e-) − (0 lone pair e-) − (1/2 x 2 bond pair e-) = 0
The sum of the formal charges of each atom equals the overall charge of the molecule or ion. As a result, in the case of NH3, each nitrogen and hydrogen has a formal charge of zero. When the charges are added together, the overall charge is zero, which is consistent with the NH3 molecule’s overall neutral charge.
Arrangement of atoms in NH3
The ammonia molecule is a trigonal pyramidal inorganic compound.
It is made up of three hydrogen atoms and a pair of unshared electrons attached to a nitrogen atom.
It is highly associated due to strong intermolecular hydrogen bonding.
It is classified as a polar molecule. When three hydrogen atoms interact with one nitrogen atom, the compound ammonia is formed.
Three hydrogen atoms and one nitrogen atom combine to form NH3, with the hydrogen atoms having electrical properties similar to helium and the nitrogen atom having electrical properties similar to neon.
By sharing electrons, the NH3 molecule is held together by the strong N–H nitrogen–hydrogen single covalent bonds. The bond angle between H–N–H is 107o.
Molecular Geometry of NH3
NH3 molecular geometry is trigonal pyramidal.
A single non-bonding lone pair of electrons on the nitrogen atom acts as a repulsive force on the bonding orbitals.
Three hydrogen atoms are linked to a nitrogen atom in the center.
Since nitrogen has five electrons in its valence shell, it must interact with three hydrogen atoms to meet the octet rule and generate ammonia, a stable molecule.
Molar Mass of NH3
Molar mass of Nitrogen = 14.0067 g/mol.
Hydrogen Molar mass = 1.00794 g/mol.
Molar mass of Ammonia (NH3)= 14.0067 g/mol + (3× 1.00794) g/mol = =17.03052 g/mol
=17.03052 g/mol
Summary
- NH3 is a neutral compound therefore, the charge of NH3 is zero.
- The NH3 oxidation number is the sum of individual oxidation numbers of the atoms nitrogen (oxidation number = -3) and hydrogen (oxidation number = 1) is zero.
- NH3 molecular geometry is trigonal pyramidal.
Related Topics
CO2 Lewis Structure and Molecular Geometry
HCN Lewis Structure| Step By Step Construction
SiO2 Lewis Structure
N2O Lewis Structure| Laughing Gas
Frequently Asked Questions
1. What is the oxidation number of NH3?
Ammonia is made up of one nitrogen atom and three hydrogen atoms (NH3). The NH3 oxidation number is zero because the sum of the individual oxidation values of the atoms nitrogen (oxidation number = -3) and hydrogen (oxidation number = 1) is zero.
2. NH3 is a polar or nonpolar molecule?
Ammonia (NH3) is a polar molecule. Since the molecule contains three N-H bond dipoles that do not cancel out. In each bond, nitrogen is more electronegative than hydrogen. This unequal distribution of charges among both nitrogen and hydrogen atoms makes NH3 a polar molecule.
3. Calcium electronic configuration?
Calcium is a silvery-white, soft metal that tarnishes rapidly in the air and reacts with water. It is the fifth most abundant metal in the Earth’s crust (4.1%) and is not found uncombined in nature but occurs abundantly as limestone (calcium carbonate), gypsum (calcium sulfate), fluorite (calcium fluoride), and apatite (calcium chloro- or fluoro-phosphate).
calcium electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2
4. How many electrons does helium have?
In a single Helium atom, there are 2 protons, 2 electrons, and 2 neutrons. Helium is the second element of the periodic table. Because s subshells can hold up to two electrons, both electrons fit into the 1s subshell; thus, the electron configuration for helium atoms is 1s2.
Check the full article “How many electrons does Helium have?”.
5. What is malleability?
Malleability refers to a substance’s ability to distort under pressure. The material can be pounded or rolled into thin sheets if it is pliable. Metal malleability refers to the ability of a metal to deform under compression and take on a new shape.
6. Valence electrons in nitrogen?
Nitrogen (N) is a nonmetallic element that belongs to Periodic Group 15 [Va]. It is the most abundant element in the Earth’s atmosphere and is found in all living things. It is a colorless, odorless, and tasteless gas. Nitrogen has a total of 5 valence electrons. Nitrogen’s electrical configuration is 1s22s22p3.
7. Silicon valence electrons?
Silicon has an atomic number of 14, which means it has two electrons in the first shell, eight electrons in the second shell, and four electrons in the third shell. Silicon’s valence shell has four electrons.
8. Ph of distilled water?
The pH of distilled water is 7, making it neither acidic nor alkaline. However, distilled water is extremely environmentally sensitive due to its high purity, and even a trace amount of carbon dioxide from the air can cause it to become slightly acidic.
9. Hydro turbines?
Hydro turbines are devices used in hydroelectric power plants to transmit energy from flowing water to a rotating shaft, where it is converted into electricity. In response to the infusion of water into their blades, these turbines revolve or spin.
10. Gamma decay?
The discharge of a gamma-ray photon, a form of high-energy electromagnetic radiation, as a result of the radioactive decay of a nucleus is referred to as gamma decay. The energy spectrum is frequently in the region of 100 keV to 10 MeV. Furthermore, unlike alpha or beta decay, no particles are ejected from the nucleus during this kind of decay.
11. The molar mass of methanol?
The molar mass of methanol is 32.04 g/mol. The methanol formula is CH3OH and it is also known as methyl alcohol, wood alcohol, or wood spirit.
12. What is proportionality constant?
The proportionality constant is a mathematical and physical term that describes a linear connection between two numbers or variables. If one variable doubles in size, the other does as well; if one variable drop in size, the other does as well. The proportionality sign looks like a stretched-out lowercase Greek letter alpha.
More Links
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023