The acetyl group is made up of a single carbonyl group linked to a methyl group.
A carbon atom is double-bonded to an oxygen atom in the Carbonyl group.
A methyl group, on the other hand, is just a carbon with three hydrogens attached (CH3). Acetic acid (CH3COOH) is an example of this group.
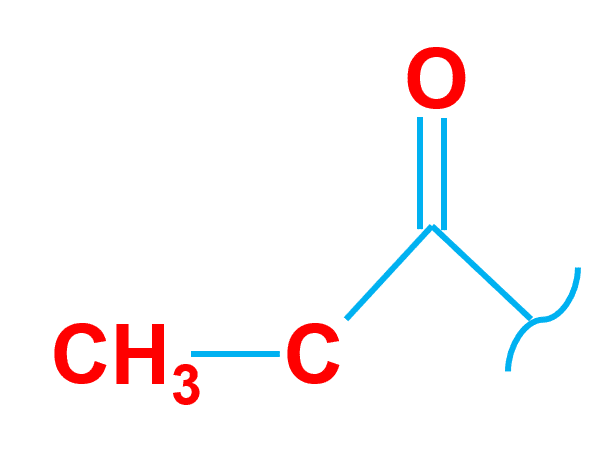
| Group Name | Acetyl Group |
| Structure of group | combination of a carbonyl group and methyl group |
| Example | Acetic acid (CH3COOH) |
| Uses | Synthesis of medicines and acetylation of wood. |
Table of Contents
What is the Carbonyl group?
A carbonyl group is a functional group comprised of a carbon atom double-bonded to an oxygen atom. A compound containing a carbonyl group refers to as a carbonyl compound.
The figure below shows a carbonyl structure, where the letter R represents the “Rest of the molecule”.
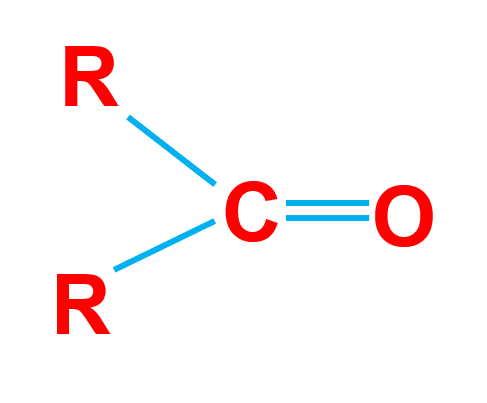
Reactivity of Carbonyl Group
- The Carbonyl group contains sigma and pi bonds so it can undergo addition reactions.
- The oxygen atom in the carbonyl group is more electronegative than the carbon atom.
It tends to attract the pi electrons to itself, making the carbonyl group a polar group. - The oxygen atom has a partial negative charge on it and it is nucleophilic whereas the carbon atom has a partial positive charge and it is electrophilic.
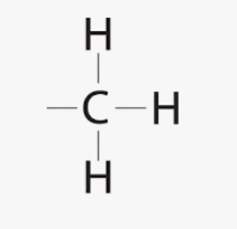
What is Methyl Group?
A Methyl group is a highly stable group, consisting of a central carbon atom covalently bonded to three hydrogen atoms. It is one of the commonest structural units of organic compounds.
The figure below shows a methyl group
Acetyl Group- Key Points
- Derived from acetic acid by dehydroxylation.
- Methyl group single-bonded to a carbonyl group
- Its chemical formula is CH3CO
- Used in the synthesis of aspirin.
- It’s the organic group of acetic acid (CH3CO-)
- It is a part of a larger family of the acyl group.
What is Acetylation?
Acetylation is the chemical process of adding the CH3CO group to a chemical compound. It is the substitution of the CH3CO with an active hydrogen atom.
When the hydrogen atom belonging to an alcohol group is replaced with an acetyl group in an acetylation reaction, an ester is formed as the product.
Examples of Acetylation
- Synthesis of Aspirin
- Acetylation reaction of aniline along with acetic anhydride for the production of acetanilide.
- Wood acetylation
Acetylation of Wood
The Acetylation of the wood is a process that involves changing free hydroxyls within the wood into acetyl groups. This is done by reacting the wood with acetic anhydride, which is taken from acetic acid. As a result, the structure of wood is permanently modified.
Naturally existing free hydroxyls groups in wood absorb and release water in accordance with the surrounding climate. The digestion of wood by enzymes initiates at the free hydroxyl sites, which is one of the reasons why wood is prone to fungal decay.
The ability of wood to absorb water decreases when hydroxyl groups transform into acetyl groups. Therefore, wood is no longer digestible by wood-destroying fungi, very durable.
Related Links
N2O Lewis Structure| Laughing Gas
SO2 (Sulfur Dioxide) Lewis structure
HCN Lewis Structure| Step By Step Construction
SiO2 Lewis Structure| Step By Step Construction
CO2 Lewis Structure and Molecular Geometry
Acetic Acid
Acetic acid is the most important carboxylic acid.
Its dilute solution is known as vinegar.
It is a colorless liquid with a boiling point of 118 degrees celsius.
It has a strong vinegar odor and sour taste.
The pure acetic acid freezes to an ice-like solid at 17 degrees celsius.
Therefore, it is also called glacial acetic acid.
It is miscible with water, alcohol, and ether in all proportions.
Uses of Acetic Acid
- Acetic acid is used as a coagulant for latex in the rubber industry.
- It is used in the manufacture of plastics (polyvinyl acetate) rayon (cellulose acetate) and silk.
- It is used in medicine as a local irritant.
- solvents in the laboratory contain acetic acid.
- Pickles contain acetic acid.
- It is used in the manufacture of many organic compounds acetone, acetates, and esters.
Frequently Asked Questions (FAQs)
1. What is the acetyl group definition?
The acetyl group consists of a carbonyl group made up of a carbon double bonded to oxygen and a methyl group, which is simply a carbon with three hydrogens (CH3). It is a part of a larger family of the acyl group
2. Is an acetyl group a ketone?
Both groups contain carbonyl groups. However, a ketone has to have carbon on both sides of the carbonyl group. An acetyl group just needs a methyl group on one side and a carbonyl group on another side.
3. What is the acetyl group?
The acetyl group consists of two parts. A first part is a carbonyl group made up of a carbon double bonded to oxygen. A second part is a methyl group, which is simply a carbon with three hydrogens (CH3).
4. What is the molar mass of acetic acid?
The molar mass of acetic acid is 60.052 g/mol. The acetic acid has the formula CH3COOH and it is also known as Ethanoic acid. The pH of a 1M solution of CH3COOH is 2.4.
5. Is SiCl4 polar or nonpolar?
SiCl4 (silicon tetrachloride) is a nonpolar molecule. Because the four chemical bonds between silicon and chlorine are uniformly distributed, SiCl4 is non-polar. A polar covalent bond is a type of covalent link that is intermediate between pure covalent bonds and ionic bonds. When the difference in electronegativity between the anion and the cation is between 0.4 and 1.7, such bonds occur.
Check the full article “Is SiCl4 polar or nonpolar?”.
6. specific heat of air?
The specific heat of air at constant pressure is 1.005 kJ/kg K and the specific heat of air at constant volume is 0.718 kJ/kg K.
The specific heat (C), also called heat capacity, of a substance is the amount of heat required to raise its temperature by one degree.
7. hydrogen ion?
A hydrogen ion is the nucleus of a hydrogen atom that has been detached from its electron. The hydrogen nucleus is made up of a proton, which is a particle with a unit positive electric charge. As a result, the solitary hydrogen ion, indicated by the symbol H+, is widely employed to represent a proton.
8. Sublimation?
Sublimation is the process by which a solid turns straight into a gaseous state without first going through the liquid stage. Dry ice is one of several sublimation instances (frozen carbon dioxide). When dry ice is exposed to air, it immediately changes its phase from solid to gaseous (fog).
Vapor deposition is the inverse of sublimation.
9. Distillation?
Distillation is the separation of a mixture of liquids based on variations in their boiling points (or volatility). Water may be extracted from a salt solution using this method.
10. nitric acid?
Nitric acid (HNO3) is a colorless, fuming, and very corrosive liquid that is employed as a laboratory reagent as well as an important industrial chemical in the manufacture of fertilizers and explosives. It is toxic and can result in severe burns.
More Links
Is NH3 Polar or Nonpolar?
Is HCl Polar or Nonpolar?
CH4 Polarity
SO2 Polar or Nonpolar
Co2 Polar or Nonpolar
Magnesium Element| Properties and Uses
Is Titanium Magnetic?
Hydrogen Bond| Definition & Easy Explanation
Is Hydrogen a Metal?
Hydrogen Electronegativity
Bottom Line
This article explains the definition, structure, and properties of the acetyl group along with details about the acetylation process. If you have any questions, please post a comment. For more related articles please visit whatsinsgith.org.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023



