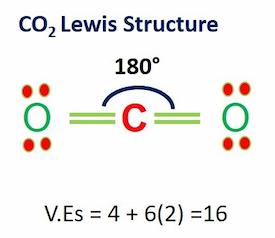
Carbon dioxide (CO2) is a colorless, odorless, incombustible gas resulting from the oxidation of carbon. Its Lewis structure comprises two different atoms: carbon and oxygen. It is a nonpolar molecule with a bond angle of 180 degrees. CO2 is used as the refrigerant in fire extinguishers and it is a significant greenhouse gas in the Earth’s atmosphere.
| Name of molecule | Carbon dioxide |
| Bond Angles | 180 degrees |
| Molecular Geometry of CO2 | Linear |
| Hybridization of CO2 | sp hybridization |
| No of Valence Electrons in the molecule | 16 |
| C-O bond distance | 115 pm |
| The dipole moment of CO2 | zero |
Table of Contents
Step By Step Construction of Lewis Structure
In a CO2 molecule, the overall ratio of carbon to the oxygen atom is 1:2. Two oxygen atoms are located on the terminals where both these atoms share electrons and form bonds with the central carbon atom.
Step-1: Count the valence electrons of atoms
To draw Lewis’s structure, we need to figure out the number of valence electrons in individual atoms as shown in the table.
| Atom | Electronic Configuration | Valence Electrons (VEs) |
| 8O | 1S2 2S2 2P4 | 6 |
| 12C | 1s2 2s2 2p2 | 4 |
VEs in carbon dioxide = VEs in 1 carbon atom + VEs in 2 oxygen atoms
VEs = 1(4)+2(6) = 16
Step-2: Determine the central atom
As per rule, C is placed in the middle because it’s the least electronegative.
If we check the proper arrangement of carbon and oxygen in the periodic table, we will find that C is less electronegative than an oxygen atom.
Put C in the center and then the oxygen on either side.
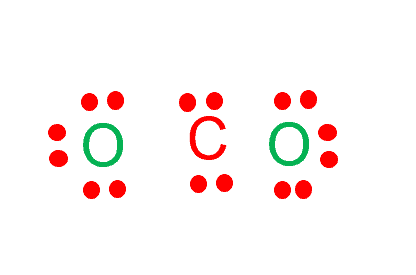
Step-3: Place electron pairs between the atoms
Draw four dots around the carbon atom.
Place two oxygen atoms on both sides of the atom and draw six dots around each atom to represent their valence electrons.
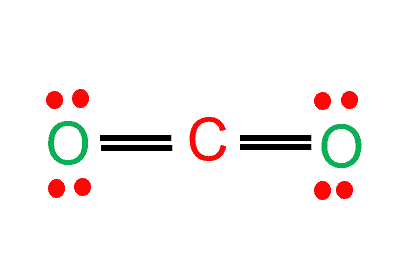
Step 4: Complete octet of the molecule
To complete the octet of the molecule, the carbon atom will donate its electrons to both oxygen atoms to form a double bond. The carbon atom will donate its electrons to oxygen atoms as they are more electronegative. Now that you know how the carbon dioxide Lewis structure is drawn, let us quickly look at the CO2 molecular geometry.
CO2 Molecular Geometry
In carbon dioxide molecular geometry, carbon forms a double bond to each of the two oxygen atoms to produce a small symmetrical, linear molecule of CO2 which is volatile and reasonably reactive.
CO2 has a linear molecular geometry because oxygen atoms form sigma bonds with the central carbon atom to complete their octet. As a result, there are no lone pairs of electrons, but bonding pairs of electrons also repel each other. Due to these repulsive forces between the valence shell electron pairs, the molecule acquires a linear shape.

How many bonds can carbon form?
Carbon can form four bonds.
Carbon’s outer shell contains four electrons. As a result, it has the ability to establish four covalent bonds with other atoms or molecules. For instance, in the CH4 lewis structure, we can notice that carbon forms four bonds with hydrogen atoms.
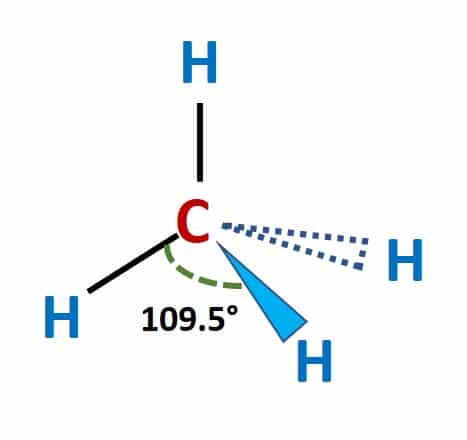
CO2 Molecular Geometry-Key Points
- CO2 exists in the gaseous state as linear molecules.
- The observed C-O bond distance is 115 pm.
- It has a face-centered cubic structure.
- Being linear, its dipole moment is zero.
Properties of Carbon dioxide
- CO2 is a nonpolar substance.
- It occurs naturally in the Earth’s atmosphere as a trace gas.
- produced during respiration by all animals, fungi, and microorganisms that depend directly or indirectly on living or decaying plants for food.
- A colorless, odorless, incombustible gas resulting from the oxidation of carbon.
- It is relatively nontoxic and noncombustible.
- It is heavier than air (53% higher density than that of dry air).
- It is used to freeze food, control chemical reactions, and is used as a refrigerant in fire extinguishers.
- It is the most significant greenhouse gas in the Earth’s atmosphere.
- The frozen solid form of CO2, known as dry ice,
- Ammonia reacts with CO2 under pressure to form urea, an important component of fertilizers and plastics.
Molar Mass
Molar mass of C =12.01 g/mol.
O2 molar mass = 16.00 x 2 = 32.00 g/mol.
Molar mass of CO2 = 44.01 g/mol
CO2 Lewis Structure (Key Points)
- Linear geometry ( linear triatomic molecule)
- The bond angle is 180 °C.
- The CO2 Lewis structure shows that it has no lone pairs of electrons.
- Carbon is in the central position as it is the least electronegative.
- The valence electrons of oxygen atoms are 6
- Valene electrons of carbon atom = 4
- The carbon-oxygen bond length is 116.3 pm
CO2 Formal Charge
Formal charge = valence electrons – unbonded electrons – 0.5 bonded electrons
Valence electrons in carbon = 4 electrons
Unbonded electrons in carbon = 0 electrons
Half of the bonded electrons in carbon =8/2 =4 electrons
Formal charge on carbon = 4–0–4 = 0
Valence electrons in central oxygen = 6 electrons.
Unbonded electrons in central oxygen = 4 electrons
Half of the bonded electrons in central oxygen =4/2 =2 electrons.
Formal charge on oxygen =6 – 4–2 =0
The formal charge on CO2 is zero. That’s why CO2 is not negative or positive.
Resonance Structure for CO2
Resonance structures are a set of two or more Lewis structures that collectively describe the delocalization of electrons of a single polyatomic ion or molecule. Resonance structures are capable of describing delocalized electrons that cannot be expressed by a single Lewis formula with an integer number of covalent bonds.
There are three resonance structures for CO2, as shown in the figure.
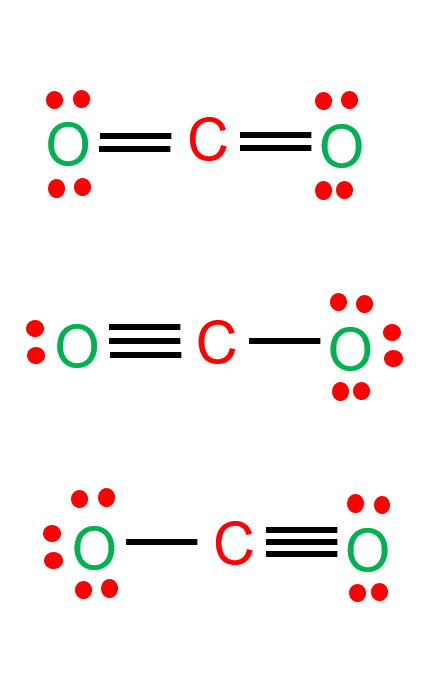
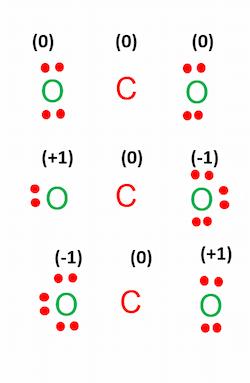
Each of the structures shown in the figure has 16 valence electrons, and all of them are valid carbon dioxide Lewis structures. Each atom in all three structures has eight electrons around it. However, there is one that is more likely or favorable. To find the most favorable structure, we need to find the formal charge of each structure, as shown in the figure below. The structure with formal charges close to zero is the most favorable Lewis structure of CO2.
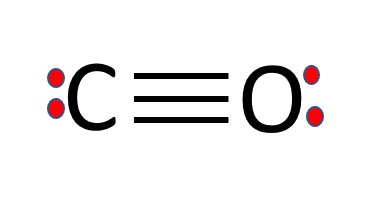
The Lewis Structure of CO
Quick steps to draw the CO lewis structure are listed below:
- Count the valence electrons: There are 10 valence electrons.
- Decide the central atom: The central atom is carbon, as it is the least electronegative.
- Place electron pairs between atoms. Draw one bond line for each electron pair. Now we have eight remaining electrons.
- Complete the octet: Draw two more bonds between the oxygen and carbon atoms.
This will use four more electrons. Now we have four remaining electrons. - To complete the octets of both these atoms, place two valence electrons on both atoms.
Why is CO2 nonpolar while CO is polar?
CO (Carbon monoxide) is polar because of the electronegativity difference between C(2.55) and O(3.44) atoms.
Both atoms have unequal charge distribution, and therefore the CO bond has a net dipole moment, making it a polar molecule.
On the other hand, CO2 is nonpolar because it has a linear, symmetrical structure.
The 2 oxygen atoms have equal electronegativity, pulling the electron density from carbon at an angle of 180 degrees from either direction.
Since there’s no unequal sharing of valence electrons in the case of carbon dioxide, it is nonpolar.
Summary
- Carbon dioxide molecules consist of a carbon atom covalently double bonded to two oxygen atoms.
- In CO2 molecules, the C-O length is 116.3 pm.
- There are three resonance structures for CO2.
- The formal charge on CO2 is zero.
- There is no lone pair of electrons in the molecule of carbon dioxide.
- Molar mass of CO2 = 44.01 g/mol
Related Links
N2O Lewis Structure
HCN Lewis Structure
SO2 Lewis structure
SiO2 Lewis Structure
O2 Lewis Structure & Molecular Geometry
Is carbon dioxide a pure substance?
Frequently Asked Questions
The following are some of the frequently asked questions. If you have any questions related to this blog, please feel free to comment.
1. Is Carbon dioxide acidic or basic?
Carbon dioxide is not an acid itself, since it does not contain ions of hydrogen (H+).
However, if CO2 is dissolved in water, it becomes carbonic acid, which is a weak acid.
2. Explain the CO2 Lewis structure in simple words.
In the carbon dioxide Lewis structure, the carbon atom is in the central position as it is the least electronegative atom in the molecule. Two oxygen atoms are located on the terminals where both these atoms share electrons and form bonds with the central carbon atom.
3. Does CO2 have lone pairs?
There are no lone pairs of electrons in CO2.
The lone pair of electrons are unshared valence electrons.
They are also called nonbonding pairs.
Lone electrons are found in the outermost electron shell of atoms.
They can be identified by using a Lewis structure.
4. What is carbon monoxide (CO)?
Carbon monoxide (CO) is a poisonous gas that has no odor or color. There is a triple bond between the carbon and oxygen atoms in the CO Lewis structure. In a CO molecule, there are ten valence electrons.
5. What is a valence electron?
A valence electron is an atom’s electron that can be transferred to or shared with another atom and is found in the atom’s outermost shell.
6. What is a Carbon footprint?
A carbon footprint, according to the World Health Organization, is a measurement of the influence of our activities on the earth’s natural greenhouse. Carbon emissions are mostly caused by human activities such as the burning of fossil fuels, deforestation, and land-use changes, which result in an increase in greenhouse gas concentrations in the atmosphere. To find out more, check out the article “How to Reduce Carbon footprint.”
7. Is carbon dioxide a pure substance?
Carbon dioxide is a pure substance since its composition remains constant regardless of where it is collected. Carbon dioxide is a greenhouse gas that is naturally occurring and innocuous in small amounts, but as levels grow, it can have an impact on productivity and sleep. CO2 levels concentrate inside with less ventilation since they are most typically created by the air we exhale.
Each carbon dioxide molecule will always have one carbon and two oxygens.
Check out the full article “Is carbon dioxide is a pure substance?”.
8. What is perchloric acid used for?
Perchloric acid (HCLO4) is used to separate potassium from sodium in many laboratory tests and industrial processes. It is also used as an oxidizing agent, especially in the determination of chromium in steel, ferrochrome, chromite, and leather.
9. NH3 oxidation number?
The NH3 oxidation number is the sum of individual oxidation numbers of the atoms nitrogen (oxidation number =-3) and hydrogen (oxidation number =1) and is zero.
10. What is nitrogen trifluoride gas?
Nitrogen trifluoride (NF3) is an odorless, colorless gas. When inhaled, it is exceedingly poisonous. Containers may burst violently if exposed to fire or heat for a lengthy period of time.
More Links
NH3 Lewis Structure & Molecular Geometry
Molar Mass of Aluminum
Weight of water
Molar Mass of Acetic Acid
N2 Lewis Structure| Hybridization & Molecular Geometry
Is BF3 Polar or Nonpolar?
| Is HCl Polar or Nonpolar? | Oxalic acid |
| How Many Neutrons Does Hydrogen Have? | Is Chlorine a metal? |
| Sublimation Examples | CH4 Polarity |
Author
Umair Javed
Umair has been working at Whatsinsight since 2020 as a content writer.
He has a Master’s degree in Materials Science.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023



