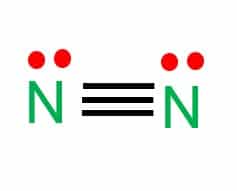
Nitrogen (N2) is a colorless, odorless, tasteless gas and is the most abundant element in Earth’s atmosphere. The N2 Lewis structure would comprise two nitrogen (N) atoms bonded together by a triple bond. Each nitrogen atom is surrounded by a lone pair of electrons.
| Name of molecule | Nitrogen |
| Bond Angles | 180 degrees |
| Molecular Geometry of Nitrogen | Linear |
| The polarity of the N2 molecule | Nonpolar |
| N2 valence electrons | 10 |
Table of Contents
Step by Step Construction of Lewis Structure
Following are the steps to construct the Lewis Structure.
Step-1: Count the valence electrons of atoms
To draw the Lewis structure, we need to figure out the number of valence electrons in individual atoms as shown in the table below.
| Atom | Electronic Configuration | Valence Electrons (VEs) |
| 7N | 1s2 2s2 2p3 | 5 |
Number of valence electrons in N2 = 5+5 = 10
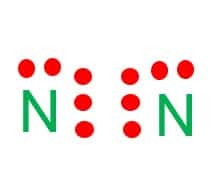
Step-2: Place electron pairs between the atoms
Both the atoms have the same electronegativity value, so there will be no central atom in the structure.
We need to arrange 10 valence electrons in the structure.
Assign the valence electrons using dots in a diagram to each atom-like 5 dots around each atom.
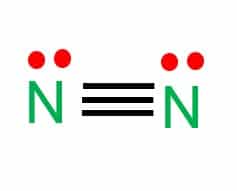
Step-3: Place remaining electrons around the other atoms
Draw lines to set up the covalent bond between both the Nitrogen atoms next to each other. One line represents one single bond.
Each atom completes its octet (eight electrons per atom) by sharing three pairs of electrons that make up the distribution of six electrons in a bond.
Each nitrogen atom contains two remaining electrons, which are called lone pairs of electrons.
Molecular Geometry
Nitrogen is a diatomic nonpolar molecule with a bond angle of 180 degrees.
Being a linear diatomic molecule, both atoms have an equal influence on the shared bonded electrons that make it a nonpolar molecule.
Lewis Structure Of N2-Key Points
- In the Lewis structure of N2, there is a triple bond between two nitrogen atoms.
- The molecular geometry of N2 is linear.
- N2 is a colorless, odorless, and tasteless gas.
- The number of electrons in the valence shell of a nitrogen atom is 5.
- Each nitrogen atom is surrounded by a lone pair of electrons.
- The molar mass of nitrogen is 28 atomic mass units.
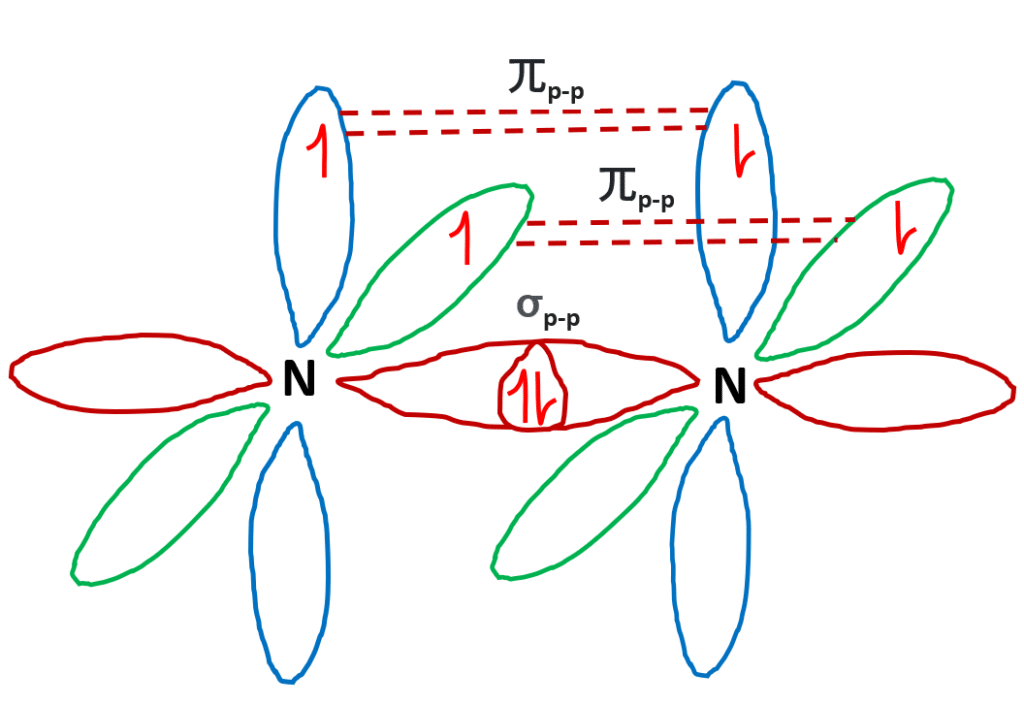
Hybridization of Nitrogen (N2)
- The electronic configuration of the N2 atom (Z =7) is 1s2 2s2 2px12py12pz1
- There are three half-filled 2p orbitals in the valence shell of the nitrogen atom.
- In the formation of the n2 molecule, one of the three half-filled 2p orbitals of each nitrogen atom overlaps mutually along the internuclear axis to form a bond.
- The remaining two half-filled 2p orbitals undergo sidewise overlapping with their parallel oriented 2p orbitals to form pi bonds.
- Therefore, two nitrogen atoms link together through triple bonds (one sigma and two pi bonds).

Uses of Nitrogen
- Most living things need n2 to survive. Nitrogen helps organisms grow, reproduce, and turn food into energy.
- Nitrogen is important to the chemical industry. It is used to make fertilizers, nitric acid, nylon, and dyes.
- Liquid nitrogen is utilized as a refrigerant for transporting foodstuffs and freezing purposes.
Is N2 Polar or Nonpolar?
N2 is a nonpolar molecule because of its linear geometrical structure and it is a diatomic molecule. As a result, both atoms have equal electronegativity and share an equal proportion of charge, and the overall molecule results in a net-zero dipole moment, making it a nonpolar molecule.
Related Links
CO2 Lewis Structure and Molecular Geometry
SiO2 Lewis Structure
SO2 (Sulfur Dioxide) Lewis structure
SO2 Lewis Structure| 4 Simple Steps
NH3 Lewis Structure & Molecular Geometry
Is Nh3 Polar?
BF3 Lewis structure| Molecular geometry, Hybridization
What is Nitrous Oxide?
Nitrous oxide (N2O) is also known as “laughing gas.” It is important for a variety of medical applications because of its anesthetic use. It is insoluble in water and works as a powerful oxidizer at higher temperatures. It’s a colorless gas with a slightly sweet odor. It can cause a narcotic effect at higher concentrations.
If a mixture of nitrogen oxide and a little oxygen is inhaled for a sufficiently long time, it produces hysterical laughter. Nitrogen oxide is also known as “laughing gas”. That’s why nitrous oxide is called “laughing gas.”
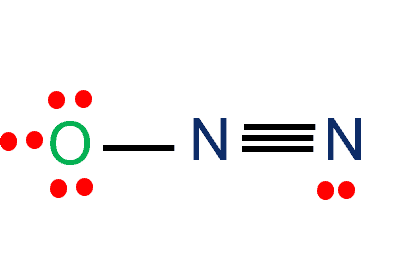
N2O Lewis Structure
In the N2o lewis structure, nitrogen (N) and oxygen (O) atoms are covalently bonded. The number of valence electrons in N and O is five and six, respectively. The total number of valence electrons in N2O is 16.
General Characteristics of Nitrogen
- Nitrogen is present in the free state in the air as a major constituent (78% by volume).
- It is a non-metal and a poor conductor of heat and electricity.
- Its compounds are covalent in nature.
- N2 is an inactive gas in comparison with oxygen, which is the next major constituent of air
- Inorganic compounds of nitrogen are not commonly found as minerals.
- In its combined state, nitrogen is found in all living matter, including, animals and plants, in the form of proteins, urea, and amino acids.
Summary
To summarize everything in this article, the following are some important points:
- In a nitrogen molecule, a triple covalent bond is represented by three lines between two atoms of nitrogen.
- The bond angle is 180 degrees, and there are 10 valence electrons.
- N2 is a nonpolar molecule with linear geometry.
Frequently Asked Questions (FAQs)
Some of the frequently asked questions are given below. If you have any other questions please feel free to comment.
1. Why Lewis structure is important?
A Lewis Structure of a Molecule is a very simplified representation of the valence shell electrons.
It shows how the electrons are arranged around individual atoms in a molecule.
Electrons are represented as “dots” or “bonding electrons” as a line between the two atoms.
The objective of drawing the Lewis structure is to obtain the “best” electron configuration, i.e. the octet rule and formal charges need to be satisfied.
2. What is the electronegativity of atoms?
Electronegativity is defined as an atom’s tendency to attract electrons to itself in a chemical bond.
The greater the difference between atom electronegativity values, the more polar the chemical bond formed between them.
- The electronegativity value increases moving from left to right across a period.
- Electronegativity generally decreases as you move down a periodic table group.
This correlates with the increased distance between the nucleus and the valence electron.
3. What are polar and nonpolar bonds?
Polar molecules are formed when the electronegativity of the bonded atoms differs. When electrons are shared equally between atoms in a diatomic molecule or when polar bonds in a larger molecule cancel each other out, nonpolar molecules form.
4. Why is nitrous oxide called “laughing gas”?
If a mixture of nitrogen oxide and a little oxygen is inhaled for a sufficiently long time, it produces hysterical laughter. Hence, nitrogen oxide is also known as “laughing gas”. That’s why nitrous oxide is called a laughing gas.
5. Explain the N2 dot structure in the simplest form
The N2 dot structure would comprise two atoms of nitrogen (N) atoms. There is a triple bond between both nitrogen atoms.
Each N is surrounded by two dots, which are called lone pairs of electrons.
It is a diatomic nonpolar molecule with a bond angle of 180 degrees.
6. Explain o2 lewis structure in the simplest form
Two oxygen atoms are joined by a double bond in the O2 Lewis structure. There are 12 valence electrons, and the bond angle is 180 degrees. O2 is a nonpolar, linear-geometry molecule.
7. Is BF3 Polar or Nonpolar?
BF3 is a non-polar compound. In BF3, the central boron atom has sp2 hybridized orbitals, resulting in an unfilled p orbital on the Bron atom and trigonal planar molecular geometry. Because the boron-fluorine bonds are all 120 degrees apart, any net dipole in that plane is canceled out. Even if each B-F bond is polar, the net dipole moment is zero because adding the bond vectors cancels everything out.
Check out the full article here“Is BF3 polar or nonpolar?”.
More Interested Links
H2S Lewis Structure & Molecular Geometry
CO Lewis Structure & Molecular Geometry
O2 Lewis Structure & Molecular Geometry
CH4 Lewis Structure & Molecular Geometry
Perchloric acid| Is It Super Acid?
HCN Lewis Structure & Molecular Geometry
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023