Oxygen (O2) is a nonpolar molecule with a zero dipole moment.
Oxygen (O2) is a diatomic, colorless, odorless, and tasteless gas with 180-degree bond angles. The molecule is made up of two oxygen atoms linked together in a pair. Because many species rely on molecular oxygen to breathe, it is critical to their existence. Oxygen (in the form of compressed gas) is also widely employed as an oxidizer in welding, metal cutting, and rocket engines.
Keep reading to know details about the questions “Is O2 polar or nonpolar?”.
| Name of molecule | Oxygen |
| Bond Angles | 180 degrees |
| Molecular Geometry of Oxygen | Linear |
| The polarity of the O2 molecule | nonpolar |
| No of Valence Electrons in the O2 molecule | 12 |
is O2 Polar or Non-Polar? The oxygen (O2) molecule is nonpolar because the molecule is diatomic and both atoms have identical electronegativity. Both oxygen atoms share equal charges and there are no partial charges on any atom. Therefore O2 is a nonpolar molecule with a zero dipole moment.
Table of Contents
O2 Molecular Geometry
Oxygen is a diatomic molecule with a linear molecular structure and 180-degree bond angles.
Both oxygen atoms in the O2 molecule have identical electronegativity, and both atoms share equal ratios of bound shared electrons, resulting in a nonpolar molecule.
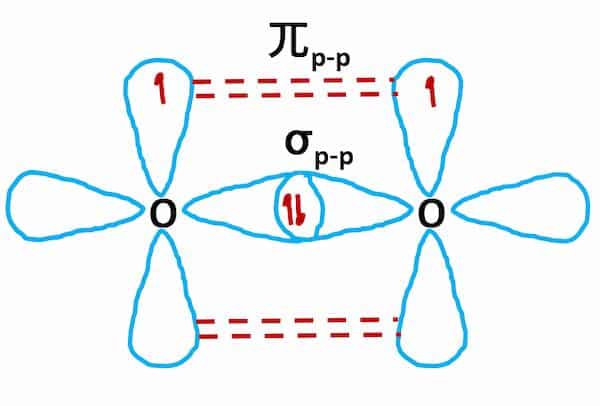
O2 Hybridization
- The electronic configuration of the O2 atom (Z=8) is 1s22s22p4
- There are two half-filled 2p orbitals in the valence shell.
- One of each oxygen atom’s two half-filled 2p orbitals overlaps along the internuclear axis to create a sigma bond. during the creation of the O2 molecule.
- To create pi bonds, the remaining half-filled 2p orbitals undergo sidewise overlapping.
- Therefore, two oxygen atoms link together through a double bond (one sigma and one pi bond)
Summary
The oxygen (O2) molecule is nonpolar because it is diatomic and both atoms have similar electronegativity. Both oxygen atoms have equal charges, and there are no partial charges on either atom. As a result, O2 is a nonpolar molecule with a zero dipole moment.
Frequently Asked Questions (FAQs)
1. How to draw the lewis structure of SO2?
SO2 Lewis structure would comprise two atoms of oxygen (O) and one sulfur atom. The number of valence electrons in both S and O atoms is six. The total number of SO2 valence electrons is 12.
- The molecular geometry of SO2 is a trigonal planner.
- The three pairs of bonding electrons are arranged in the plane at an angle of 120-degree.
- The sulfur’s valence electron = 6
- valence electrons of oxygen in SO2 are 6
2. What are unbonded pairs of electrons?
Unbonded pairs of electrons are unshared valence electrons.
They are also called lone pairs of electrons.
They are found in the outermost electron shell of atoms and can be identified by drawing lewis’s structure.
3. What is SO2?
SO2 (Sulfur dioxide) is the entity of a bond between Sulfur and Oxygen atoms.
It is a colorless, toxic, and inorganic gas with a pungent smell like Nitric acid.
SO2 gives a weak acid solution when dissolved in water.
It is naturally found in small amounts in the atmosphere and is a primary precursor of Sulfuric acid.
4. What are the uses of oxygen?
Steel, plastics, and textiles production, steel and other metals brazing, welding, and cutting, rocket propellant, oxygen therapy, and life support systems in airplanes, submarines, spaceflight, and diving are all examples of common oxygen applications.
5. Is BF3 Polar or Nonpolar?
BF3 is a non-polar molecule. The central boron atom in BF3 contains sp2 hybridized orbitals, resulting in an empty p orbital and trigonal planar molecular geometry. Any net dipole in that plane is canceled out since the Boron-Fluorine bonds are all 120 degrees apart. Even though each B-F bond is polar, the net dipole moment is zero since the bond vectors cancel out. Check the full article “Is BF3 polar or nonpolar?”
6. NH3 is a polar or nonpolar molecule?
Ammonia (NH3) is a polar molecule. Since the molecule contains three N-H bond dipoles that do not cancel out. In each bond, nitrogen is more electronegative than hydrogen. This unequal distribution of charges among both nitrogen and hydrogen atoms makes NH3 a polar molecule.
7. NH3 oxidation number?
The NH3 oxidation number is the sum of individual oxidation numbers of the atoms nitrogen (oxidation number = -3) and hydrogen (oxidation number = 1) is zero.
8. What is nitrogen trifluoride gas?
Nitrogen trifluoride (NF3) is an odorless, colorless gas. When inhaled, it is exceedingly poisonous. Containers may burst violently if exposed to fire or heat for a lengthy period of time.
9. What is perchloric acid used for?
Perchloric acid (HCLO4) is used to separate potassium from sodium in many laboratory tests and industrial processes. It is also used as an oxidizing agent, especially in the determination of chromium in steel, ferrochrome, chromite, and leather.
10. CH4 polar or nonpolar?
Methane (CH4) is a nonpolar molecule.
Related Links
CO2 Lewis Structure and Molecular Geometry
SO2 (Sulfur Dioxide) Lewis structure
N2O Lewis Structure| Laughing Gas
Is HCN Polar or Nonpolar?| Simple Explanation
SO2 Polar or Nonpolar
SiCl4 Polar or Nonpolar
Ionic Compounds| Properties, and Examples
Is MgCl2 Ionic or Covalent?
SO2 Ionic or Covalent?
Is Ammonium Ion (NH4+) Polar or Nonpolar?
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023

