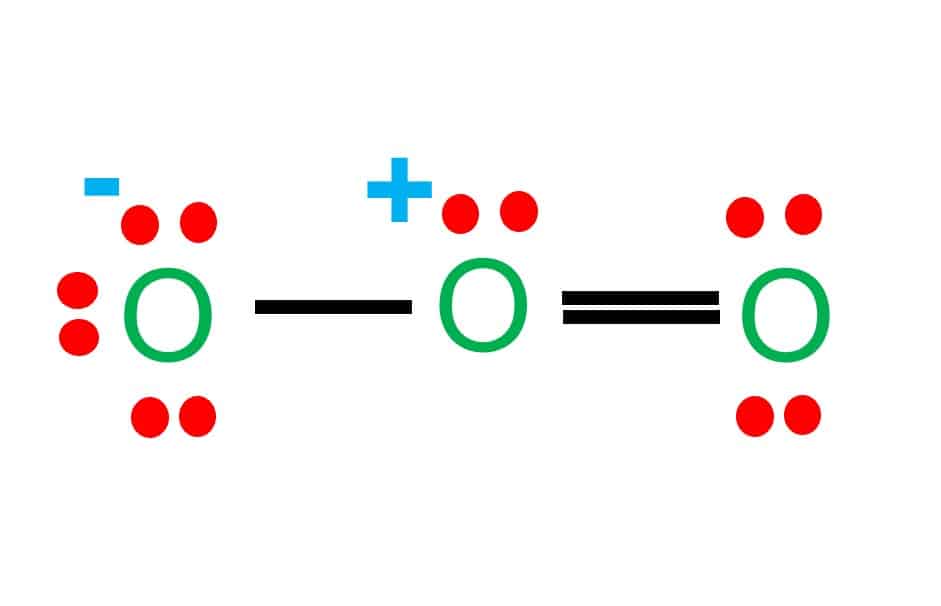
Oxygen (O2) is a diatomic, colorless, odorless, tasteless gas with bond angles of 180 degrees. O2 Lewis structure comprises two oxygen atoms connected in a pair. Many species require molecular oxygen for breathing, making it vital for life. Oxygen (as a compressed gas) is also frequently employed in welding, metal cutting, and rocket engines as an oxidizer.
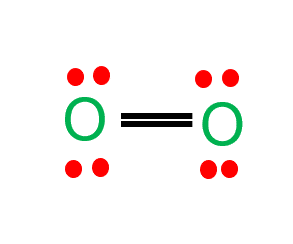
| Name of molecule | Oxygen |
| Bond Angles | 180 degrees |
| Molecular Geometry of Oxygen | Linear |
| The polarity of the O2 molecule | nonpolar |
| No of Valence Electrons in O2 molecule | 12 |
Table of Contents
Step by Step Construction of Lewis Structure
The following are the steps to constructing the Lewis Structure.
Step-1: Count the valence electrons of atoms
To draw the Lewis structure, we need to figure out the number of valence electrons in individual atoms as shown in the table below.
| Atom | Electronic Configuration | Valence Electrons (VEs) |
| 8O | 1S2 2S2 2P4 | 6 |
Valence electrons in O2 = 6+6 = 12

Step-2: Place electron pairs between the atoms
The Lewis diagram of O2 shows two oxygen atoms having twelve dots, of valence electrons. Where six are arranged, around each oxygen atom
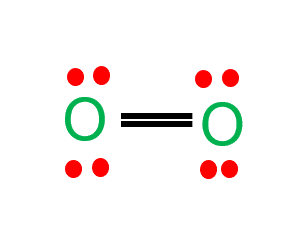
Step-3: Place remaining electrons around the other atoms
As the oxygen atom requires only two valence electrons, it readily shares electrons with the neighboring oxygen atom to complete its octet.
These four valence electrons form two shared pairs of covalent bonds, providing a stable structure for the oxygen molecule.
The resulting bond is a covalent bond.
O2 Molecular Geometry
Oxygen is a diatomic molecule with linear molecular geometry and bond angles of 180 degrees.
In the O2 molecule, both oxygen atoms have equal electronegativity and both atoms share equal ratios of bonded shared electrons, and the overall molecule turns out to be nonpolar in nature.
O2 Lewis Structure- Key Points
- In the O2 Lewis structure, there is a double bond between two oxygen atoms.
- O2 is a colorless, odorless, and tasteless gas.
- Density = 1.429 g/L
- The boiling point is -183.0 C.
- Melting point 218.4 °C
- The molar mass of O2 is 15.9994 g/mol.
- Oxygen has two allotropic forms, O2 and O3.
- The O2 molecule is nonpolar in nature.

Uses of Oxygen
- Most living things need oxygen to survive. Oxygen helps organisms grow, reproduce, and turn food into energy.
- The production of steel depends upon oxygen, which is used in a blast furnace to turn carbon into carbon dioxide, which reduces the iron oxides to pure iron.
- Oxygen is used in torches for cutting and welding.
- Oxygen can be heated to over 5,000 degrees by reacting with hydrogen. This hot mixture can cut through or weld together most metallic substances.
Is O2 polar or nonpolar?
O2 is a nonpolar molecule.
In the O2 molecule, both oxygen atoms equally share the 4 electrons that make up the double bond between them. Equal electronegativity means that there are no partial charges for each element. Since neither atom pulls harder, it’s a non-polar covalent bond.
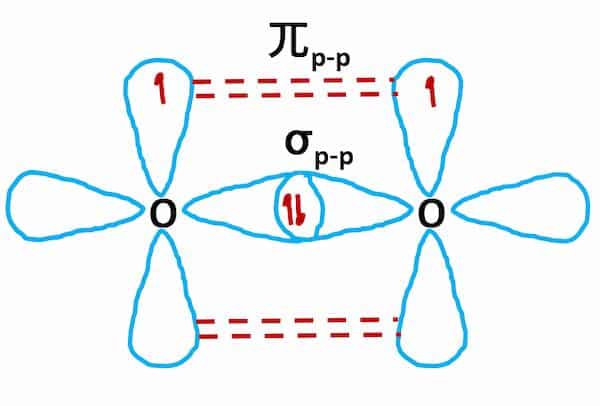
Hybridization of Oxygen (O2)
- The electronic configuration of the O2 atom (Z = 8) is 1s22s22p4.
- There are two half-filled 2p orbitals in the valence shell.
- In the formation of the O2 molecule, one of the two half-filled 2p orbitals of each oxygen atom overlaps mutually along the internuclear axis to form a sigma bond.
- The remaining half-filled 2p orbitals undergo sidewise overlapping to form pi bonds.
- Therefore, two oxygen atoms are linked together through a double bond (one sigma and one pi bond)
Oxygen Characteristics
- At room temperature, oxygen is a non-metal element that exists as a gas. It has two oxygen atoms in each of its molecules.
- Oxygen comprises about 50% of the earth’s crust.
- Respiration, the process of transferring energy from glucose to cells, requires oxygen.
- It is a gas at room temperature, and about 1/4 of the atmosphere by weight consists of oxygen.
- Water contains nearly 89% of combined oxygen.
- Calcium carbonate, which occurs in chalk and limestone marble, contains 48% oxygen.
- Silica, which is found in flint quartz, contains more than 50% oxygen.
- It is sparingly soluble in water and does not react with water.
- It is paramagnetic in nature and has two oxidation states.
- Burning requires the presence of oxygen. However, burning occurs only when the mixture of fuel and oxygen is sufficiently heated.
Summary
To summarize everything in this article, the following are some important points:
- In the O2 Lewis structure, two oxygen atoms are connected together with a double bond.
- The bond angle is 180 degrees, and there are 12 valence electrons.
- O2 is a nonpolar molecule with linear geometry.
- There are four lone electron pairs in the oxygen molecule.
Related Links
CO2 Lewis Structure and Molecular Geometry
SO2 (Sulfur Dioxide) Lewis structure
N2O Lewis Structure| Laughing Gas
CLF3 Lewis Structure, Molecular Geometry, and Polarity
How Many Water Bottles is a Gallon| Examples
HCN Lewis Structure & Molecular Geometry
Frequently Asked Questions (FAQs)
1. How to draw the Lewis structure of SO2?
SO2 Lewis structure would comprise two atoms of oxygen (O) and one sulfur atom. The number of valence electrons in both S and O atoms is six. The total number of SO2 valence electrons is 12.
- The molecular geometry of SO2 is a trigonal planner.
- The three pairs of bonding electrons are arranged in the plane at an angle of 120 degrees.
- The sulfur valence electron is equal to 6.
- The valence electrons of oxygen in SO2 are 6
2. What are unbonded pairs of electrons?
Unbonded pairs of electrons are unshared valence electrons.
They are also called lone pairs of electrons.
They are found in the outermost electron shell of atoms and can be identified by drawing lewis’s structure.
3. What is SO2?
SO2 (Sulfur dioxide) is the entity of a bond between Sulfur and Oxygen atoms.
It is a colorless, toxic, and inorganic gas with a pungent smell like Nitric acid.
SO2 gives a weak acid solution when dissolved in water.
It is naturally found in small amounts in the atmosphere and is a primary precursor of Sulfuric acid.
4. What are the uses of oxygen?
Production of steel, plastics, and textiles, brazing, welding, and cutting of steel and other metals, rocket propellant, oxygen therapy, and life support systems in airplanes, submarines, spaceflight, and diving are all examples of common applications of oxygen.
5. How many electrons does oxygen have?
A single oxygen atom has eight protons, eight electrons, and eight neutrons.
Oxygen is a stable isotope of oxygen with a nucleus of 8 neutrons and 8 protons. Its mass is 15.99491461956 u. Check the full topic “How many electrons does oxygen have?”.
6. What is the density of oxygen?
The density (ρ) of oxygen (O2) is 1.428 g/L at standard temperature and pressure. The molar mass of oxygen is 32 grams per mole.
One mole of a gas at STP (0°C and 1 atm) has a volume of 22.4 L. So if we have the molar mass of the gas, just divide it by 22.4 to get the density of that gas. The molar mass of O2 gas = 32 g/mol.
Density = mass/volume
Density of oxygen = (32 g/mol) / (22.4 L/mol) = 1.428 g/L
More Links
Because of its linear, symmetrical form, carbon dioxide (CO2) is nonpolar. The electron density is drawn equally from both sides by the two oxygen atoms in either direction of the carbon atom. CO2 is nonpolar in nature because there is no uneven sharing of valence electrons.
The gas sulfur dioxide (SO2) has a polar character. It’s a polar molecule because of the electronegativity mismatch between the sulfur (2.58) and oxygen (3.44) atoms. Additionally, SO2 has a bent shape due to the existence of unbonded electrons on the sulfur and oxygen atoms.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023