The oxygen formula is O2. Oxygen is a diatomic, colorless, odorless, and tasteless gas with 180-degree bond angles. The Oxygen formula comprises two oxygen atoms connected in a pair. Many species rely on oxygen to breathe, making it essential for survival. Oxygen (as a compressed gas) is also commonly used as an oxidizer in welding, metal cutting, and rocket engines. Some of the traits of oxygen are given in the table below:
| Name of molecule | Oxygen |
| Bond Angles | 180 degrees |
| Molecular Geometry of Oxygen | Linear |
| The polarity of the O2 molecule | nonpolar |
| No of Valence Electrons in O2 molecule | 12 |
| Molar Mass | 15.999 u |
| Density of oxygen | At STP = 1.429 g/L When Liquid = 1.141 g/cm3 |
| Melting Point | −218.79 °C |
| Boiling Point | −182.962 °C |
| Electronic configuration | 1s22s22p4 |
Table of Contents
Oxygen Chemical Formula
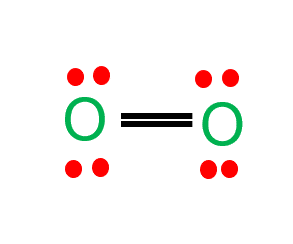
If we analyze the oxygen formula O2, There are two oxygen atoms in an oxygen molecule. Each Oxygen with six valence electrons shares two electrons to form two covalent bonds, one sigma bond, and the other pi bond.
The axial overlap of 2p atomic orbitals of oxygen forms the sigma bond, and the lateral overlap of 2p atomic orbitals of oxygen forms the pi bond. Both oxygen atoms in an oxygen molecule have completed their octet. A representation of the oxygen structural formula is given below.
Oxygen Characteristics
- Oxygen is a nonmetal element that exists as a gas at room temperature.
- About half of the earth’s crust is made up of oxygen.
- At room temperature, it is a gas, and it makes up roughly one-quarter of the weight of the atmosphere’s air.
- Water contains nearly 89% of combined oxygen.
- Calcium carbonate which occurs as chalks, limestone marble contains 48% oxygen.
- Silica which is found in flint quartz contains more than 50% oxygen.
- It is sparingly soluble in water and does not react with water.
- Oxygen is paramagnetic in nature and has two oxidation states.
- Combustion requires the presence of oxygen. However, burning occurs only when the mixture of fuel and oxygen is sufficiently heated.
O2 Molecular Geometry
Oxygen is a diatomic molecule with linear molecular geometry and 180-degree bond angles.
Both oxygen atoms in the O2 molecule have equal electronegativity, and both atoms share equal ratios of bonded shared electrons, resulting in a nonpolar molecule. Check the full article “O2 lewis structure and molecular geometry”.
Why Oxygen is Important?
- Most living things need O2 to survive. Oxygen helps organisms grow, reproduce, and turn food into energy.
- Respiration, the process of transferring energy from glucose to cells, necessitates the presence of oxygen.
- The production of steel depends upon oxygen which is used in a blast furnace to turn carbon into carbon dioxide, which reduces the iron oxides to pure iron.
- Oxygen is used in torches for cutting and welding.
- Oxygen can be heated to over 5,000 degrees by reacting with hydrogen. This hot mixture can cut through or weld together most metallic substances.
The polarity of Oxygen Molecule
O2 is a nonpolar molecule. O2 is a nonpolar molecule. The four electrons that make up the double bond between the oxygen atoms in the O2 molecule are shared equally by both of them. Equal electronegativity means that each element has no partial charges. It’s a non-polar covalent bond because neither atom pulls harder than the other.
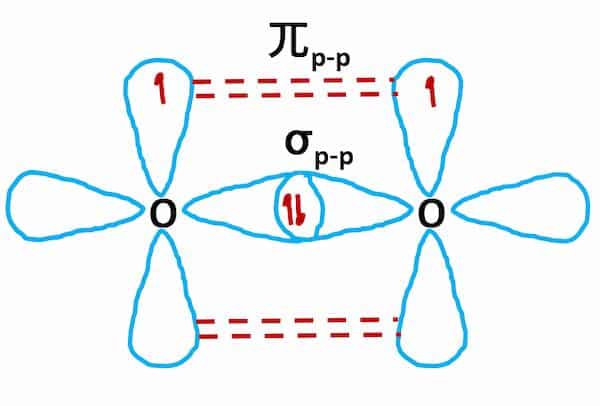
Hybridization of O2 Molecule
- The electronic configuration of the O2 atom (Z=8) is 1s22s22p4
- The valence shell contains two half-filled 2p orbitals.
- One of each oxygen atom’s two half-filled 2p orbitals overlaps mutually along the internuclear axis to form a sigma bond during the formation of the O2 molecule.
- To form pi bonds, the remaining half-filled 2p orbitals overlap sideways.
- As a result, a double bond forms between two oxygen atoms (one sigma and one pi bond)
Summary
To summarize everything in this article, the following are some important points:
- The oxygen formula is O2
- In the O2 Lewis structure, two oxygen atoms are connected together with a double bond.
- The bond angle is 180 degrees and there are 12 valence electrons.
- O2 is a nonpolar molecule with linear geometry.
- There are four lone pairs of electrons in the oxygen molecule.
Related Links
Is Water a Mixture?
How many hydrogen atoms are in a molecule of water?
Hydrogen oxide-An Overview
Is Air a Homogeneous Mixture?
Frequently Asked Questions (FAQs)
1. What is the temperature of liquid nitrogen?
Liquid oxygen is extremely cold and boils at –297.3 degrees Fahrenheit. It is a liquified form of oxygen gas that is used as an oxidant for liquid fuels in missile and rocket propellant systems. Although it is not flammable, it is a powerful oxidizer.
2. What is the number of electrons in oxygen?
A single oxygen atom has eight protons, eight electrons, and eight neutrons.
Oxygen is a stable isotope of oxygen with a nucleus of 8 neutrons and 8 protons. Its mass is 15.99491461956 u. Check full topic “How many electrons does oxygen have?”.
3. What is the density of oxygen?
The density (ρ) of oxygen (O2) is 1.428 g/L at standard temperature and pressure. The molar mass of oxygen is 32 grams per mole. One mole of a gas at STP (0°C and 1 atm) has a volume of 22.4 L. So if we have the molar mass of the gas, just divide it by 22.4 to get the density of that gas. The molar mass of O2 gas = 32 g/mol.
Density = mass/volume
Density of oxygen = ( 32g/mol) / (22.4 L/mol) = 1.428 g/L
4. What is Sulfur dioxide?
Sulfur dioxide is the result of a bond between the atoms of sulfur and oxygen.
It is a colorless, toxic, inorganic gas with a pungent odor similar to nitric acid.
When SO2 is dissolved in water, it produces a weak acid solution.
It is a primary precursor of sulfuric acid and is naturally found in small amounts in the atmosphere.
5. What are the uses of oxygen?
Production of steel, plastics, and textiles, brazing, welding, and cutting of steel and other metals, rocket propellant, oxygen therapy, and life support systems in airplanes, submarines, spaceflight, and diving are all examples of common applications of oxygen.
6. How does oxygen participate in a combustion reaction?
A combustion reaction is a chemical reaction that produces both heat and light. The most common type of combustion is fire. When the gas oxygen reacts with another substance, the majority of combustion occurs. For example, when wood burns, oxygen in the air combines with carbon in the wood.
7. Is helium a gas?
Helium (He) is an inert gas and chemical element in Periodic Group 18. (noble gases). Helium, the second lightest element (only hydrogen is lighter), is a colorless, odorless, and tasteless gas that freezes at 268.9 degrees Celsius (452 degrees Fahrenheit).
8. At what temperature does water freeze?
Water’s normal freezing and melting points are 0 degrees Celsius or 32 degrees Fahrenheit.
9. Hydrogen cyanide polar or nonpolar?
HCN is a polar molecule.
As can be seen from the HCN lewis structure, the electronegativity difference between nitrogen (3.04) and hydrogen (2.2) makes it a polar molecule.
More Links
SiO2 Lewis Structure| Step By Step Construction
Why is Water a Polar Molecule?
CO Lewis Structure & Molecular Geometry
Is Carbon Dioxide a Pure Substance?
Ideal Gas Law| Simple Overview
Photonics
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023