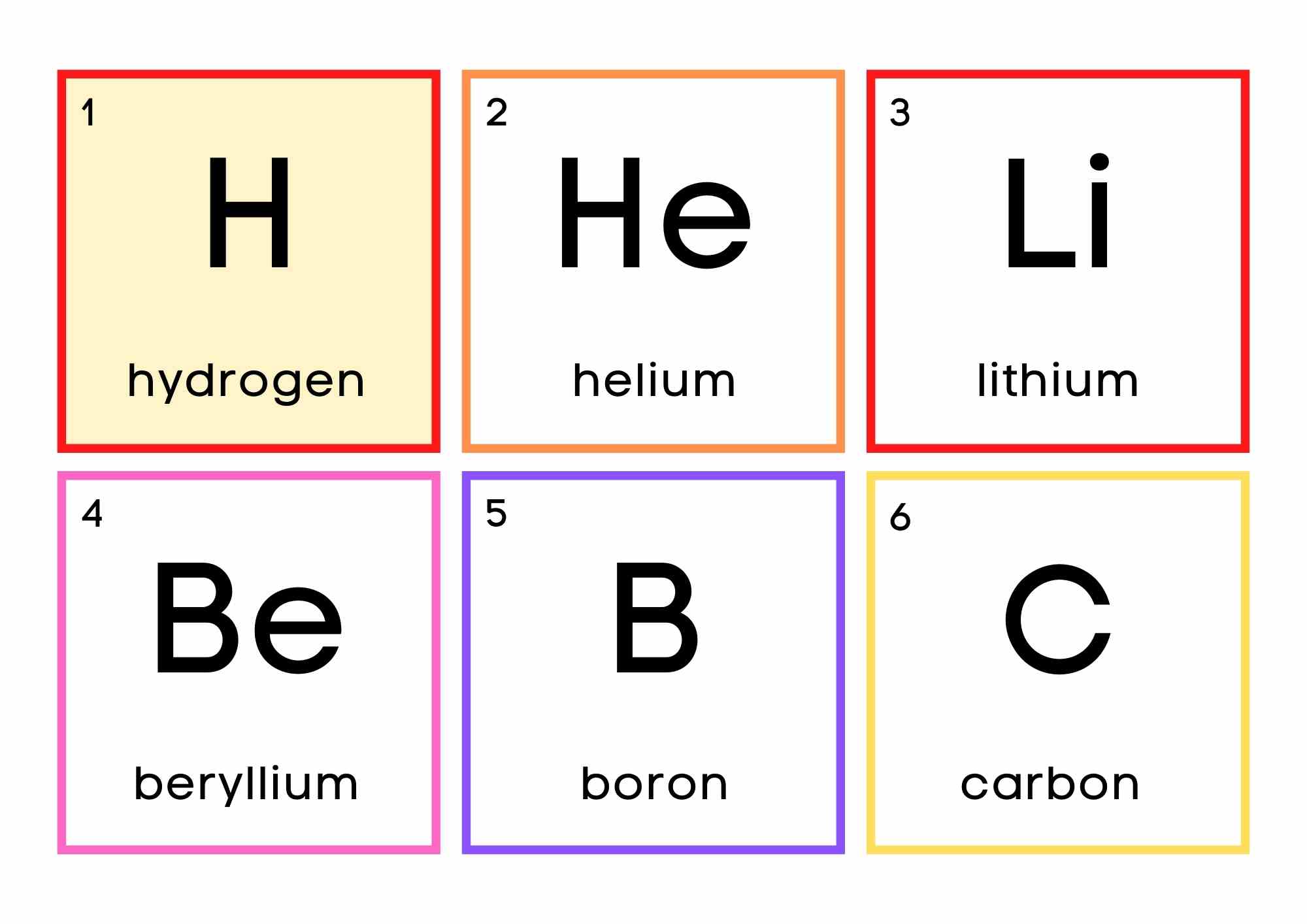
Hydrogen is a nonmetal that is placed above the alkali metals in the periodic table due to its ns1 electron configuration.
Hydrogen (H) is a colorless, odorless, tasteless, flammable gaseous substance that is the most fundamental member of the chemical element family. The hydrogen atom has a nucleus composed of a proton with one unit of positive electrical charge and an electron with one unit of negative electrical charge. Hydrogen gas is a loose aggregation of hydrogen molecules, each of which consists of two atoms, a diatomic molecule, H2. While hydrogen has the same ns1 electron configuration as alkali metals, it is not metal because it behaves differently than alkali metals because it forms cations (H+) more slowly than other alkali metals. Therefore, the answer to the question “is hydrogen a metal?” is simply no.
Table of Contents
Hydrogen Molar Mass
The molar mass of hydrogen is 1.00794 g/mol. The molar mass of an element or molecule is the total mass in grams of all the atoms in a mole of a certain molecule. Because hydrogen does not exist as a single atom, the hydrogen molecule has a molar mass of 2.016 g/mol.
Why gases are non-metals?
Gases lack the qualities of metallic elements, such as metallic bonding, conductivity, ductility, brilliance, and other metallic properties.
Related Links
Hydrogen Ion | Definition, Charge & Formula
Hydrogen oxide-An Overview
Hydrogen Iodide
Sodium Hydrogen Sulfate
Hydrogen Phosphate Formula
Lewis Structures| Hydrogen (H2), and Water (H2O)
Bottom Line
The answer to the question, ” Is hydrogen a metal?”, is hydrogen is a nonmetal as it is a flammable gaseous substance.
Frequently Asked Question
1. Is MgCl2 Ionic or Covalent?
When the magnesium atom loses two electrons to create the Mg2+ ion and each chlorine receives one electron to form the Cl– ion, an ionic connection is formed between the magnesium and chlorine atoms.
Check “Is MgCl2 ionic or covalent?”.
2. Is SiCl4 polar or nonpolar?
SiCl4 (silicon tetrachloride) is a nonpolar molecule. Because the four chemical bonds between silicon and chlorine are uniformly distributed, SiCl4 is non-polar. A polar covalent bond is a type of covalent link that is intermediate between pure covalent bonds and ionic bonds. When the difference in electronegativity between the anion and the cation is between 0.4 and 1.7, such bonds occur.
Check the full article “Is SiCl4 polar or nonpolar?”.
3. Is chlorine a metal?
Chlorine is a nonmetal. Metals are malleable and are good heat and electricity conductors. Non-metals are (usually) poor conductors of heat and electricity, and they are not malleable or ductile; many of the elemental non-metals are gases at room temperature. Furthermore, Chlorine is an oxidant that accepts electrons, so it has the properties of a nonmetal.
4. How many valence electrons are in nitrogen?
Nitrogen has a total of five valence electrons (as seen in the Lewis Dot structure of nitrogen). Phosphorus, Arsenic, Antimony, and Bismuth are other elements with five valence electrons.
5. How many neutrons does hydrogen have?
The majority of hydrogen atoms lack a neutron. Rare hydrogen isotopes, known as deuterium and tritium, have one and two neutrons, respectively. Check the full article “How many neutrons does hydrogen have?”.
6. What is a hydrogen bond?
The hydrogen bond is a chemical bond formed by the hydrogen atom and more electronegative elements such as N, O, and F. For instance, HOH, NH3, and so on. In water, a hydrogen bond is a dynamic attraction between neighboring water molecules that involves one hydrogen atom located between two oxygen atoms.
7. How many hydrogen atoms are in a molecule of water?
Individual H2O molecules are V-shaped and are made up of two hydrogen atoms that are attached to the sides of a single oxygen atom. Because oxygen atoms are electronegative, they attract the shared electrons in covalent bonds. As a result, the electrons in a water molecule spend slightly more time around the oxygen atomic center and slightly less time around the hydrogen atomic center. As a result, the covalent bonds are polar, and the oxygen atoms have a slight negative charge (due to the presence of an extra electron share), while the hydrogens have a slightly positive charge (from the extra un-neutralized protons). Check the full article “How many hydrogen atoms are in a molecule of water?”.
8. H2S is polar or nonpolar?
Because of the presence of a lone pair of electrons in Sulfur and the electronegativity difference between Sulfur and H atoms, hydrogen sulfide is polar.
More Interesting Links
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023