The sublimation process is a solid-to-gas transition. One of the many sublimation examples is dry ice (frozen carbon dioxide). When dry ice gets exposed to air, it directly changes its phase from a solid state to a gaseous state (fog).
Sublimation is the opposite of vapor deposition.
This article covers the sublimation definition, process, examples, and case study.
Table of Contents
Sublimation Examples

- Water cycle
- Dry ice
- Naphthalene Mothballs that chase moths away (quite a common sublimation example)
- Frozen foods leave behind ice crystals inside a bag.
- Solid air fresheners are sublime in nature.
- Sublimation printing
- It is also employed to purify volatile solids contaminated with non-volatile impurities.
- For example, tea is also used to create freeze-dried substances, for example, tea, soup, or drugs, by a process called lyophilization
Key Points

- Solids turn directly into gases.
- Endothermic process
- a physical change, not a chemical change.
- It’s not a vaporization process.
- used for the purification of solids.
What is the sublimation definition in chemistry?
Sublimation is defined as a process in which a solid directly changes into its gaseous state without passing through the liquid state.
Generally, the conversion of a solid to a gaseous state requires an intermediate liquid state.
However, in sublimation, solid molecules directly change to a gaseous state by absorbing heat.
This process relates to a physical change of state, and it does not involve a chemical reaction.
For instance, the dissociation on heating of solid ammonium chloride into hydrogen chloride is a chemical reaction and is not a sublimation example.
Sublimation example (Ice to water vapors)
When snowfall turns directly into water vapor without first transitioning into a liquid, it is called sublimation. The southern parts of Mount Everest are the best places to witness this process.
Conversion of dry ice to gas
If you drop a solid piece of dry ice (solid carbon dioxide) into water. Carbon dioxide changes directly to gas vapors without first melting into a liquid. As a result, dry ice creates a smoky effect that is commonly used in ice cream parlors.
Sublimation Printing
Sublimation printing (Dye-sublimation example) is a handy process for printing high-quality images on mugs, cell phone cases, tiles, trophies, and apparel. The CAD design is printed using a special printer, which is very similar to an inkjet printer. However, it uses specific ink cartridges. The printed image is placed on the surface to be sublimated, between the top and bottom of the heat press. Pressure and heat are applied to turn the printed image into gas. The ink penetrates the pores of the substrate. After that, the ink gets solidified and creates a permanent image of the product.
Separation of Ammonium Chloride from sand
Apparatus Required
Bunsen burner, Tripod stand, China dish, Cotton, Matchbox, Filter paper, Funnel, Mixture of sand and Ammonium Chloride.
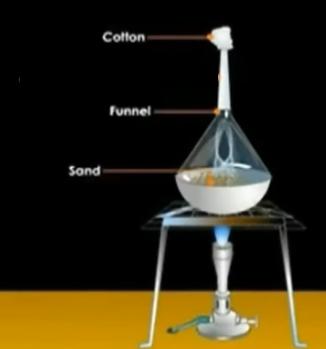
Steps
- Add the mixture in China dish.
- Place the china dish on a tripod stand containing wire gauze.
- light bunsen burner under the tripod stand.
- Place an inverted funnel over the china dish.
- Plug in a little cotton at the opening of the stem of the funnel.
- On heating the mixture in the china dish, white fumes evolve and rise inside the funnel.
- Stop heating when the white fumes stop rising and allow the funnel to cool.
- After that, remove the funnel from the china dish and, using a spatula, transfer the solid ammonium chloride sticking on the walls of the funnel into a watch glass.
Similarly, this process is used in the purification of many pharmaceutical substances such as iodine, camphor, naphthalene, benzoic acid, and mercuric chloride.
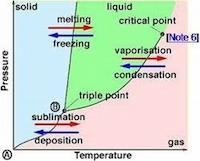
What is Triple Point?
The “triple point” is the point having a definite temperature and pressure at which the solid, liquid, and vapor phases of a chemical substance co-exist
Sublimation and Triple Point
The pressure below the triple point is called the sublimation pressure.
When the vapor pressure of the substance is less than that of sublimation pressure, it will directly change into the solid or vapor phase without changing into the liquid phase.
Sublimation is the opposite of vapor deposition.
A vapor deposition technique is a coating process in which materials in a vapor state are condensed through condensation, chemical reaction, or conversion, to deposit thin films on different substrates and is classified as physical (PVD) and chemical (CVD) vapor deposition methods.
Summary
In sublimation, a solid get directly transformed into a gas phase without going through the liquid state. This phenomenon occurs because of the absorption of heat by solid molecules. This absorbed heat helps molecules overcome the attractive forces of their neighbors and escape into the vapor phase. Dry ice and naphthalene balls are sublimation examples. Sublimation is the opposite of vapor deposition.
Frequently Asked Questions (FAQs)
1. What are sublimation examples?
- “Dry ice” or solid carbon dioxide sublimes.
- Snow and ice can sublime in the winter months without melting.
- Mothballs sublime.
- Frozen foods will sublime and you will find ice crystals inside the box or bag.
2. How are sublimation and evaporation similar?
Both produce gas as their final product. Both are endothermic.
3. What properties must a solid have to undergo sublimation?
This process occurs in solids with vapor pressures that exceed atmospheric pressure at or near room temperature.
4. Is Dye-sub printing expensive?
Dye-sub printing is a lot more expensive than ordinary printing. You need a dedicated printer because the ink can cause a jam. If you don’t use it very often, it’ll cost you a lot in printer maintenance and ink, and that specialty ink is crazy expensive because this method takes a bit more time per piece than screen printing.
5. What is a Dye-sub printer?
A dye-sub printing printer and heat-press sublimate shapes, patterns, and images onto the surface of materials such as polypropylene and textiles.
It is actually an inkjet printer, but rather than installing normal ink cartridges into the printer, special ink cartridges are installed instead. Also, once a printer is utilized for sublimation, it can no longer be used as a regular printer.
6. What are the steps for Dye-sub printing?
- Produce a design using CAD software.
- Print the design using a designated printer.
- Place printed images on the surface of your product.
- Place your product between the top and bottom plates of the hot press.
- Apply pressure and heat.
- Take away the final product.
7. Why does oil float on water?
Oil floats on water because its density is lower than that of water. Density in liquids is defined as the amount of mass that may be filled into a cubic meter of volume. Water has a density of roughly 1000 kg/cubic meter, while oil has a density ranging from 800 to 960 kg/cubic meter.
8. Heat flux?
The rate of thermal energy flow per unit surface area of a heat transfer surface, such as in a heat exchanger, is known as heat flux.
9. Calcium electron configuration?
Calcium is a silvery-white, soft metal that tarnishes rapidly in the air and reacts with water.
calcium electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2
10. How many electrons does helium have?
In a single helium atom, there are 2 protons, 2 electrons, and 2 neutrons. Helium is the second element of the periodic table. Check out the full article “How many electrons does helium have?”.
11. Valence electrons in nitrogen?
Nitrogen has 5 valence electrons. Nitrogen (N) is a non-metallic element in Periodic Group 15 [Va]. It is a colorless, odorless, and tasteless gas that is the most abundant element in the Earth’s atmosphere and is found in all living things.
More Interesting Links
Author
Ms. Sana Javed (Ph.D. scholar)
Sana has been working at Whatsinsight since 2020 as a content writer. She has a MPhil degree in pharmaceutics.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023




