A hydrogen bond (H-bond) is a dipole-dipole interaction. It is not an actual chemical bond.
It develops when a hydrogen (H) atom is bonded to a strongly electronegative atom.
(fluorine, nitrogen, or oxygen ) exists in the vicinity of another electronegative atom with a lone pair of electrons.
This type of bond is weaker than an ionic or covalent bond, but it is stronger than van der Waals forces.
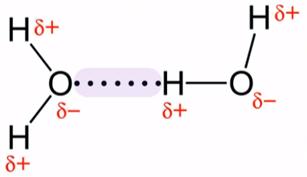
Table of Contents
Hydrogen Bond Definition
A hydrogen bond is defined as a polar covalent bond in which a hydrogen atom is attracted to a strongly electronegative atom in the same or another molecule, such as oxygen, fluorine, or nitrogen. A strong dipole-dipole attraction connects the hydrogen atom and an electronegative atom.
How Hydrogen Bond Is Formed?
In a hydrogen bond, the hydrogen atom is covalently bonded to fluorine, nitrogen, or oxygen atoms.
These elements are extremely electronegative and withdraw the bulk of electrons in their direction.
The hydrogen atom takes on a small positive charge due to the electron affinity of these atoms.
This makes the hydrogen electron deficient and results in a dipole in the molecule.
The negative end of this dipole attracts the positive end of the surrounding dipole.
As a result, a weak bond is formed between both dipoles.
This weak bond is called the hydrogen bond.
Molecules that have non-polar covalent bonds do not form H-bonds.
Examples of Hydrogen Bonding
- Water (H2O)
- Chloroform (CHCl3)
- Ammonia (NH3)
- Acetylacetone (C5H8O2)
- Hydrofluoric acid (HF)
- Alcohol
Hydrogen bonding in water
Due to extensive H-bonding, Water (H2O) is liquid over a much wider temperature spectrum than would be predicted for a molecule of its size.
Since H2O readily forms H-bonds with the solute, water is an excellent solvent for ionic compounds and many other compounds.
H-bonding contributes to the unique properties of water, including its high boiling point (100 °C) and surface tension.
The H-bond with heavy water (where the isotope of hydrogen is deuterium) is even stronger than those with normal water (where the isotope of hydrogen is tritium).
Please refer to an interesting article, “Why is water a polar molecule?”.

DNA molecules
The two complementary strands of DNA are held together by H-bonds between complementary nucleotides (A&T, C&G). For detailed DNA structure and function. For details, check the link.
H-Bonding in Ammonia
In the case of ammonia, intermolecular H-bonds form between the hydrogen of one molecule and the nitrogen of another. the resulting bond is very weak because each nitrogen has one lone electron pair.
H-Bonding in Hydrofluoric acid
Hydrofluoric acid (HF) forms a symmetric hydrogen bond, where the proton is spaced halfway between two identical atoms. An asymmetric H-bond is stronger than a regular hydrogen bond. It’s comparable to the strength of a covalent bond.
Types of Hydrogen Bonds
There are mainly two types of H-bond s, intermolecular H-bonds, and intramolecular H-bond s.
Intramolecular Type
In the intramolecular type of H bonding, two functional groups in a molecule are arranged in such a way that they can attract each other. For instance, in salicylic acid, the alcohol (-OH) group on the ring attracts the carboxylic acid group (the double-bonded oxygen).
Intermolecular Type
Intermolecular H-bonds occur when one molecule contains a partially positive hydrogen atom and the other molecule contains a partially negative atom.
This type of bonding occurs between water molecules.
It also occurs between water and alcohol and aldehyde.
Importance Of H-Bonding
H-bonding between water molecules helps maintain a stable temperature near large bodies of water.
It allows humans to cool themselves via perspiration and causes ice to float.
This type of bonding is important for biomolecules, such as DNA, cellulose, and proteins.
Role of Electronegativity in H-Bonding
- In H-bonding, the hydrogen atom is bonded to a highly electronegative atom.
The polarization is directly proportional to electronegativity.
So, hydrogen bonded to oxygen is more able to form a hydrogen bond than hydrogen bonded to carbon. - The electronegative atom must be small. The smaller the size of the atom, the greater its electrostatic attraction. So, fluorine is better at forming H-bonds than iodine.
Key Points- Hydrogen Bonding
- Hydrogen bonds are weak bonds.
- They are weaker than covalent bonds (which are, in turn, weaker than ionic bonds).
- An H-bond is about 5% the strength of the covalent O-H bond.
- Most H-bonds form between hydrogen (H) and oxygen (O), fluorine (F), or nitrogen (N).
- H-bonds are stronger than van der Waals forces.
- Molecules that experience H-bonding have higher boiling points, so they are less volatile.
- The H-bonding in alcohol increases the boiling point because extra energy is required to break the H-bonds and permit boiling.
- Ethanol and other alcohols contain H-bonds between hydrogen and oxygen.
- H-bonds are found between the repeating units of the polymer.
Related Links
Hydrogen Electronegativity
Hydrogen Ion | Definition, Charge & Formula
Hydrogen Molar Mass
Is Hydrogen a Metal?
HCL Intermolecular Forces
Physical and Chemical Properties of Water
Frequently Asked Questions
1. How do you break a hydrogen bond?
Water molecules are held together by weak H-bonds.
These bonds allow the water molecules to stick with each other.
However, by simply adding another material to the water, these bonds can be broken.
2. How is H-bond formed?
When the H atom is covalently bonded to electronegative atoms like fluorine, nitrogen, or oxygen.
A dipole is formed as electronegative atoms withdraw electrons towards them.
The positive end of this dipole gets attracted towards the negative end of the neighboring dipole.
This force of attraction is called H-bonding.
3. How many cups in a gallon?
A US liquid gallon is equal to 16 cups, and a US dry gallon is equal to 18.61 cups. In the United States, one cup equals half a pint (236.6 ml).
To get a more detailed answer, click “how many cups in a gallon.”
4. What is the polarity of NH3?
NH3 is polar because it has three nitrogen-hydrogen bond dipoles that do not cancel out. In each bond, nitrogen is more electronegative than hydrogen. The polarity comes from the unequal distribution of charges among both nitrogen and hydrogen atoms. Check the full article “Is NH3 polar?”.
5. Hydrogen cyanide polar or nonpolar?
HCN is a polar molecule.
As can be seen from the HCN Lewis structure, the electronegativity difference between nitrogen (3.04) and hydrogen (2.2) makes it a polar molecule.
6. How many neutrons does hydrogen have?
The majority of hydrogen atoms lack a neutron. Rare hydrogen isotopes, known as deuterium and tritium, have one and two neutrons, respectively. Check out the full article “How many neutrons does hydrogen have?”.
References
- Sweetman, A. M.; Jarvis, S. P.; Sang, Hongqian; Lekkas, I.; Rahe, P.; Wang, Yu; Wang, Jianbo; Champness, N.R.; Kantorovich, L.; Moriarty, P. (2014). “Mapping the force field of a hydrogen-bonded assembly”. Nature Communications. 5: 3931. doi:10.1038/ncomms4931
- Weinhold, Frank; Klein, Roger A. (2014). “What is a hydrogen bond? Resonance covalency in the supramolecular domain”. Chemistry Education Research and Practice. 15: 276–285. doi:10.1039/c4rp00030g
Related Links
Density Of Water (g/ml)
Is H2S Polar Or Nonpolar
How Many Bottles of Water Equal a Gallon
NH3 Lewis Structure & Molecular Geometry
Is BF3 Polar or Nonpolar?
H2S Lewis Structure & Molecular Geometry
Distillation Process Definition| Easy Explanation
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023



