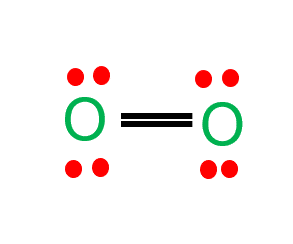
In a single oxygen atom, there are eight protons, eight electrons, and eight neutrons.
The initial two electrons in the electron configuration for oxygen will be in the 1s orbital. Because the 1s orbital can only store two electrons, the next two electrons for oxygen are placed in the 2s orbital.
As a result, the oxygen electron configuration is 1s22s22p4.
Oxygen-16 (16O) is a stable isotope of oxygen, having 8 neutrons and 8 protons in its nucleus. It has a mass of 15.99491461956 u.
| Name of molecule | Oxygen |
| Bond Angles | 180 degrees |
| Molecular Geometry of Oxygen | Linear |
| The polarity of the O2 molecule | nonpolar |
| Valence Electrons in the O2 molecule | 12 |
| The electron configuration of oxygen | 1s22s22p4 |
Table of Contents
What is oxygen?
Oxygen (O2) is a diatomic, colorless, odorless, and tasteless gas with 180-degree bond angles. The O2 Lewis structure is made up of two oxygen atoms bonded in a pair. Many species rely on molecular oxygen to breathe, making it essential for survival. Oxygen (as a compressed gas) is also commonly used as an oxidizer in welding, metal cutting, and rocket engines.
O2 molecular geometry
Oxygen is a diatomic molecule with linear molecular geometry and bond angles of 180 degrees.
In the O2 molecule, both oxygen atoms have equal electronegativity and both atoms share equal ratios of bonded shared electrons, and the overall molecule turns out to be nonpolar in nature.
Oxygen Density
The density (ρ) of oxygen (O2) is 1.428 g/L at a standard temperature and pressure. The molar mass of oxygen is 32 grams per mole.
One mole of a gas at STP (0°C and 1 atm) has a volume of 22.4 L. So if we have the molar mass of the gas, just divide it by 22.4 to get the density of that gas. The molar mass of O2 gas = 32 g/mol.
Density = mass/volume
Density of oxygen= (32 g/mol) / (22.4 L/mol) = 1.428 g/L
Summary
- The answer to the question “How many electrons does oxygen have?” is oxygen atom has eight electrons.
- Oxygen is a diatomic molecule with linear molecular geometry and bond angles of 180 degrees.
- Oxygen is also commonly used as an oxidizer in welding, metal cutting, and rocket engines.
Related Links
Co2 Polar or Nonpolar
SO2 Polar or Nonpolar
O3 Lewis Structure | Step By Step Drawing
O2 Molar Mass
O2 Lewis Structure & Molecular Geometry
Frequently Asked Questions
1. Does oxygen have 6 or 8 valence electrons?
Because oxygen is in Periodic Group VI, it possesses six valence electrons, two in the 2s subshell and four in the 2p subshell. Check out the full article on oxygen valence electrons.
2. How many electrons does oxygen have?
A single oxygen atom has eight protons, eight electrons, and eight neutrons.
Oxygen is a stable isotope of oxygen with a nucleus of 8 neutrons and 8 protons. Its mass is 15.994914.1956 u. Check the full topic “How many electrons does oxygen have?”.
More Links
How many electrons does Helium have?
How many valence electrons does sodium have?
How many valence electrons are in a silicon atom?
Titanium Electron Configuration
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023