Ideal gas law states that the pressure of gas times its volume equals the number of moles of the gas times a constant (R) times the temperature of the gas. The ideal gas law is a quite important statement of the gas laws since it relates the quantity of gas (moles) to its pressure, volume, and temperature.
Furthermore, the ideal gas law is an important tool in chemical and engineering computations involving gases. The ideal gas equation is given as:
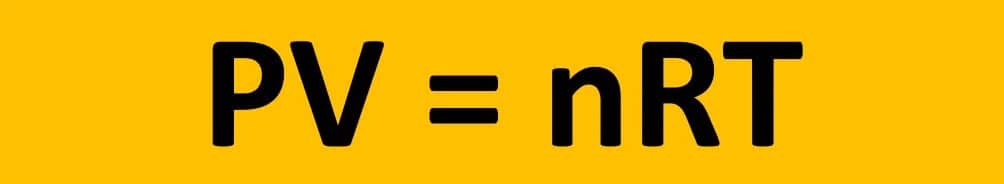
where:
- P: Pressure, measured in units of force per unit area (such as pounds per square inch or pascals)
- V: Volume, measured in units of length cubed (such as cubic meters or liters)
- n: Amount of substance, measured in units of moles (a unit of measurement for the number of atoms or molecules)
- R: The gas constant, a constant value that has units of energy per degree of temperature per mole (such as joules per kelvin per mole)
- T: Temperature, measured in units of temperature (such as kelvin or Celsius)
Table of Contents
General Gas Constant
The factor R in the ideal gas law equation is known as the “gas constant”. R=PV/nT.
In other words, the pressure multiplied by the volume of a gas divided by the number of moles and the temperature of the gas equals a constant value called gas constant. The numerical value of the gas constant is determined by the units of pressure, volume, and temperature. As a result, there are several values for R that correspond to various sets of measuring units.
| Pressure | Volume | Temperature | Gas constant |
| kPa | Liters | K | 8.314 L·kPa mol-1·K-1 |
| atm | Liters | K | 0.0821 L·atm mol-1·K-1 |
| mmHg | Liters | K | 62.4 L·mm Hg mol-1·K-1 |
Volume of 1 mole of a gas at Standard Temperature and Pressure
PV = nRT, V = nRT/P;
P = 1 atm; n = 1 mol; T = 273 K; R = 0.0821 L·atm mol-1·K-1
V = nRT/P = 1 (0.0821 L·atm mol-1·K-1)( 273 K)/1 atm = 22.4 Liters
Volume of 1 mole of a gas at standard temperature and pressure is 22.4 liters.
Ideal Gas Law Assumptions and Derivation
The ideal gas law assumes that gases behave ideally, meaning they adhere to the following characteristics:
- Collisions between molecules are elastic, and their motion is frictionless, therefore the molecules do not lose energy;
- The overall volume of the individual molecules is magnitudes lower than the volume that the gas occupies;
- The molecules are continually moving, and the space between two molecules is far greater than the size of a single molecule.
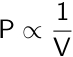 | Boyle’s Law |
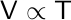 | Charle’s Law |
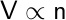 | Avagadro’s Law |
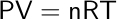 | Combining and simplifying the above equations we get the ideal gas law |
Ideal Gas law in Simple Words
- The Ideal Gas Law is a simple equation that describes the behavior of gases.
- The equation states that the pressure, volume, and temperature of a gas are related to each other in a specific way.
- The equation is written as PV = nRT, where P is the pressure of the gas, V is its volume, n is the number of gas molecules present, R is the gas constant, and T is the temperature of the gas in Kelvin.
- According to the equation, the pressure of a gas is directly proportional to its temperature and the number of gas molecules present.
- The equation also states that the pressure of a gas is inversely proportional to its volume.
- This equation can be used to make predictions about the behavior of gases under different conditions.
- The Ideal Gas Law is a fundamental principle in many areas of physics and chemistry.
Daily Life Examples of Ideal Gas Law
- Balloons: When you blow up a balloon, you’re increasing the volume of the gas inside it. According to the ideal gas law, as you increase the volume of the gas, the pressure inside the balloon decreases. This is why balloons become softer when you let out some of the air.
- Scuba Diving: When scuba divers descend to deeper depths, the pressure of the gas they’re breathing increases. This is because the water above them is exerting more pressure on the gas molecules in their tanks. The ideal gas law explains how changes in pressure affect the volume and temperature of the gas inside the tank.
- Air Conditioning: Air conditioners work by compressing a gas, such as Freon, to cool the air. As the gas is compressed, its temperature and pressure increase, according to the ideal gas law. Then, when the gas is allowed to expand, its temperature and pressure decrease, which creates cool air.
- Baking: When you bake a cake, you’re using the ideal gas law to your advantage. As the cake batter heats up in the oven, the gas molecules inside it start to expand. This causes the cake to rise and become fluffy.
- Bicycle Tires: When you pump up a bicycle tire, you’re increasing the pressure of the gas inside it. According to the ideal gas law, as you increase the pressure of the gas, its volume decreases. This is why the tire becomes firm and able to support the weight of the bicycle and rider.
Summary of Ideal Gas Law
| Definition | A physical law that describes the behavior of ideal gases under various conditions. |
| Equation | PV = nRT, where P is pressure, V is volume, n is the amount of gas (in moles), R is the gas constant, and T is temperature (in Kelvin). |
| Assumptions | The law assumes that gases are made up of a large number of tiny particles that are in constant, random motion, and that these particles are not subject to intermolecular forces. Ideal gases are also assumed to have no volume and no interactions between molecules. |
| Use | The Ideal Gas Law is used to calculate the properties of gases under different conditions, such as changes in pressure, volume, temperature, and amount. It can be used to determine the relationships between these properties, and to calculate the properties of a gas given other known values. |
| Units | The units of pressure, volume, and temperature used in the Ideal Gas Law are typically expressed in atmospheres (atm), liters (L), and Kelvin (K), respectively. The gas constant R can be expressed in a variety of units, including L·atm/(mol·K), J/(mol·K), and m³·Pa/(mol·K). |
| Limitations | The Ideal Gas Law is only applicable to ideal gases, which do not exist in the real world. Real gases have volume and are subject to intermolecular forces, which can cause them to deviate from the Ideal Gas Law under certain conditions. |
Difference between Ideal and Real Gases
| Ideal Gases | Real Gases |
| Assumes no volume and no intermolecular forces | Has volume and intermolecular forces |
| Pressure is directly proportional to number of particles | Deviations from ideal gas behavior at high pressures and low temperatures due to intermolecular forces and volume |
| Infinitely compressible | Not compressible beyond a certain point |
| Does not liquefy | Can be liquefied |
| No surface tension or viscosity | Has surface tension and viscosity |
| Constant heat capacity | Variable heat capacity |
| Constant speed of sound | Variable speed of sound |
| Obeys the Ideal Gas Law | Deviations from the Ideal Gas Law under certain conditions |
| Infinite thermal conductivity | Finite thermal conductivity |
What is Ideal gas constant in simple terms with examples?
The Ideal Gas Constant is a physical constant that relates the pressure, volume, temperature, and amount of a gas in a system. Its value is approximately 8.31 J/(mol·K) or 0.082 L·atm/(mol·K) or 1.987 cal/(mol·K), depending on the units used.
In simpler terms, the Ideal Gas Constant can be thought of as a conversion factor between different units of measurement that are used to describe gases. For example, if you know the pressure, volume, temperature, and amount of a gas in a system, you can use the Ideal Gas Constant to calculate the number of moles of gas present.
Multiple Choice Questions
- Which of the following variables is not included in the Ideal Gas Law equation?
a. Pressure
b. Volume
c. Temperature
d. Mass
Answer: d. Mass
- What is the Ideal Gas Law equation?
a. P = V/T
b. P = VnR/T
c. PV = nT/R
d. P = V/nT
Answer: c. PV = nRT
- According to the Ideal Gas Law, if the temperature of a gas is held constant, what will happen to the pressure of the gas if its volume is decreased?
a. The pressure will increase.
b. The pressure will decrease.
c. The pressure will remain the same.
d. It is impossible to determine what will happen to the pressure.
Answer: a. The pressure will increase.
- At constant pressure, the volume of a gas is directly proportional to its:
a. Temperature
b. Pressure
c. Amount
d. Density
Answer: a. Temperature
- Avogadro’s Law states that the volume of a gas is directly proportional to its:
a. Temperature
b. Pressure
c. Amount
d. Density
Answer: c. Amount
Solve Problem- Ideal Gas Law
Problem: What is the volume of 2.5 moles of nitrogen gas at a pressure of 1.5 atm and a temperature of 25°C?
Step 1:
Convert the temperature to Kelvin. The Ideal Gas Law equation requires temperature to be in Kelvin, so we need to add 273.15 to 25°C to get the temperature in Kelvin: T = 25°C + 273.15 = 298.15 K
Step 2:
Write down the given values and the Ideal Gas Law equation: n = 2.5 mol (amount of gas) P = 1.5 atm (pressure) V = ? (volume, what we want to find) R = 0.0821 L·atm/(mol·K) (gas constant) T = 298.15 K (temperature)
PV = nRT
Step 3:
Substitute the given values into the equation: (1.5 atm) V = (2.5 mol) (0.0821 L·atm/(mol·K)) (298.15 K)
Step 4:
Solve for V: V = (2.5 mol) (0.0821 L·atm/(mol·K)) (298.15 K) / (1.5 atm) V = 34.7 L
Step 5:
Round the final answer to the appropriate number of significant figures. The given pressure and temperature have three significant figures, so the final answer should also have three significant figures: V = 34.7 L (rounded to three significant figures)
Related Links
Greenhouse Effect| Definition And 5 Key Factors
Hess’s Law: Statement, Equation, & Examples
How cold is Liquid Nitrogen?
Graham’s Law – Diffusion and Effusion
Isolated System Definition| Science
Gauge Pressure Formula
Summary
The ideal gas law states that PV = NRT, where P is the absolute pressure of a gas, V is the volume it occupies, N is the number of atoms and molecules in the gas, R is a gas constant, and T is its absolute temperature. Furthermore, An ideal gas is one in which the particles do not attract or repel one another and occupy no space (have no volume).
Frequently Asked Questions
1. What is an ideal gas?
An ideal gas is a hypothetical gas whose molecules occupy negligible space and have no interactions, and hence precisely obey the gas laws. The ideal gas is a gas that obeys all of the gas laws at all temperatures and pressures.
While there are no perfect gases, many gases behave in ways that are similar to ideal gases under specific situations. The concept of an ideal gas is beneficial for understanding gas behavior and simplifying gas property calculations.
2. What is the relationship between P and V in the Ideal Gas Law?
The ideal gas Law states that PV = nRT. Robert Boyle discovered PV = is a constant. That is, the product of a gas’s pressure and volume is a constant for every given gas sample. In Boyle’s experiments, neither the temperature (T) nor the number of moles (n) of gas present changed. Please refer full article “PV-diagram”.
3. What is the ideal gas law used for?
The ideal gas law can be used to compute the volume of gases consumed or generated. The ideal-gas equation is extensively used in chemical equations to convert between volumes and molar values.
More Links
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023



