A water molecule (H2O) consists of two hydrogen atoms attached to the sides of a single oxygen atom.
A single oxygen atom has six electrons in its outer shell, which may store up to eight electrons in total. The outer electron shell of oxygen is filled when two hydrogen atoms are bonded to one oxygen atom.
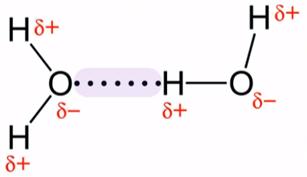
In water, the hydrogen atom, which is a little bit positive, from one water molecule is weekly attracted to an oxygen atom which is a little bit negative in a neighbouring water molecule.
Table of Contents
Weight Of Water
The weight of water depends upon the temperature at which it is stored.
For the purposes of answering the question, “How much does a gallon of water weigh?” we will focus on the weight of fresh water at 62 degrees Fahrenheit (17 degrees Celsius).
1 US gal of water = 8.345 pounds = 3.785 kg (at 17 °C).
1 Imperial gal of water = 10.02 pounds = 4.545 kg (at 17 °C).
Weight of 1 liter (l) of pure water (at 4 °C) = 1 kilogram (kg).
Density Of Water
At 4.0°C (39.2°F), the density of water in g/ml is 0.9998395. This is the same as 1 gram per milliliter (g/ml) or 1 gram per cubic centimeter (g/cm3) rounded up.
The gram per milliliter (1 g/ml), gram per cubic centimeter ( g/cm3), and pounds per cubic foot ( lb/ft3) are common water quantities. At 4°C, the density of fresh water on Earth is typically assumed to be 1000 kg/m3.
Density is the amount of matter in a certain volume. It is the mass-to-volume ratio of a substance. If we use the same amount of wood and iron. We’ll discover that iron is significantly heavier than wood. This is owing to the fact that iron has a greater density than wood.
How Many Bottles Of Water Equal A Gallon?
A gallon (gal) is 231 cubic inches, which is equal to 3.785 liters and 7.58 bottles of water in the United States. One gallon is equal to 4.55 liters and 9.1 bottles in the Imperial system used in the United Kingdom. A gallon contains 128 fluid ounces, while a half-gallon has 64 fluid ounces.
Please refer to the full article “How many bottles of water equal a gallon” for in-depth analysis.
Summary
- The answer to the question “How many hydrogen atoms are in a molecule of water?” is two.
- A water molecule (H2O) consists of two hydrogen atoms attached to the sides of a single oxygen atom.
- A single oxygen atom has six electrons in its outer shell, which may store up to eight electrons in total.
- The outer electron shell of oxygen is filled when two hydrogen atoms are bonded to one oxygen atom.
Frequently Asked Question
1. What is water molecule and how many hydrogen atoms are in a molecule of water?
Water is a substance that exists in gaseous, liquid, and solid phases and is made up of the chemical elements hydrogen and oxygen. It is one of the most abundant and necessary chemicals. At room temperature, it is a tasteless and odourless liquid with the critical capacity to dissolve many other compounds. Water’s capacity as a solvent is critical to living creatures.
A water molecule consists of two hydrogen atoms attached to the sides of a single oxygen atom.
2. Is water vapor a greenhoouse gas?
Water vapour is a major greenhouse gas because it absorbs longwave radiation and radiates it back to the surface, contributing to global warming. Furthermore, by weight and volume, it is the most abundant greenhouse gas in the atmosphere.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023



