Carbon monoxide (CO) is a poisonous gas that has no odor or color. It has two distinct atoms in its Lewis structure: carbon and hydrogen. It’s a polar molecule with 180-degree bond angles. There is a triple bond between the carbon and oxygen atoms. The CO molecule has a total of ten valence electrons.
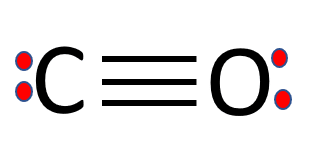
| Name of molecule | Carbon monoxide (CO) |
| Bond Angle | 180 degrees |
| Molecular Geometry of CO | Linear |
| Hybridization of CO | sp hybridization |
| No of Valence Electrons in the molecule | 10 |
| C-O bond distance | 112.8 pm |
| The dipole moment of CO | 0.122 D |
Table of Contents
Lewis Structure of CO
In a CO Lewis structure, the overall ratio of carbon to the oxygen atom is 1:1. Carbon forms three triple bonds with oxygen by sharing three valence electrons. This completes its octet. Similarly, oxygen shares three valence electrons with carbon to complete its octet. Oxygen has one lone pair of electrons as well.
Step-1: Count the valence electrons of atoms
To draw the Lewis structure, we need to figure out the number of valence electrons in individual atoms as shown in the table.
| Atom | Electronic Configuration | Valence Electrons (VEs) |
| 8O | 1S2 2S2 2P4 | 6 |
| 12C | 1s2 2s2 2p2 | 4 |
VEs in carbon monoxide = VEs in 1 carbon atom + VEs in 1 oxygen atom
VEs = 1(4)+1(6)
Valence electrons in CO = 10 electrons
Step-2: Determine the central atom
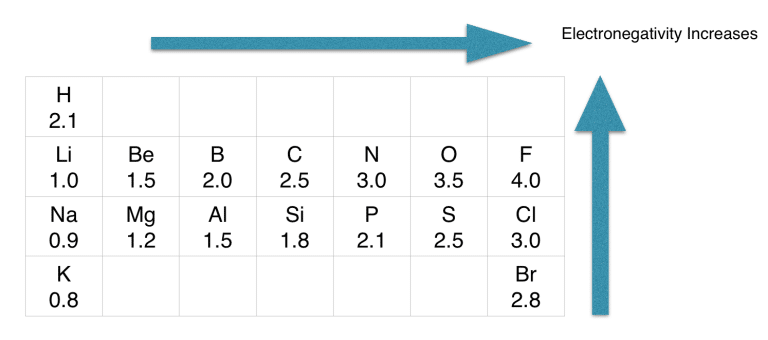
If we check the proper arrangement of Carbon (C) and oxygen (O) in the periodic table, we find that carbon (2.5) is less electronegative than the oxygen (3.5) atom.
Since there are only two atoms; place the atoms beside each other.
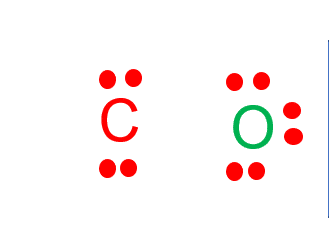
Step-3: Place electron pairs between the atoms
Draw four dots around the carbon atom.
Place two oxygen atoms on both sides of the atom and draw six dots around the oxygen atom to represent its valence electrons.
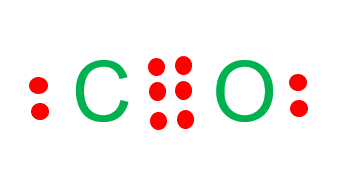
Step 4: Complete octet of the molecule
The last step is to complete the octet of the molecule.
Carbon has four valence electrons and it needs four more to complete its octet.
In contrast, oxygen has six valence electrons and it needs two more electrons to complete the octet.
Construct three bonds between oxygen and carbon atoms. This will leave four valence electrons only. To complete the octets of both atoms, place two valence electrons on both atoms.
CO Molecular Geometry
Carbon monoxide has a linear molecular geometry, a triple bond between C and O, and each atom contains one lone pair of electrons. Carbon and oxygen from one sigma bond and two pi bonds.
Properties of Carbon monoxide
- CO is a polar substance.
- It is highly poisonous, odorless, colorless, and tasteless.
- It is produced by the incomplete burning of fossil fuels.
- CO is a highly toxic gas and can cause acute illness and, in worst-case scenarios, death.
- Carbon monoxide is the most common type of fatal poisoning in the world.
Molar Mass
Molar mass of carbon =12.01 g/mol.
Oxygen molar mass = 16.00 g/mol.
Molar mass of CO = 28.01 g/mol
CO Dot Structure (Key Points)
- Linear geometry ( linear diatomic molecule)
- The bond angle is 180 °C.
- Two lone pairs of electrons
- Valence electrons of oxygen atom =6
- Valene electrons of carbon atom = 4
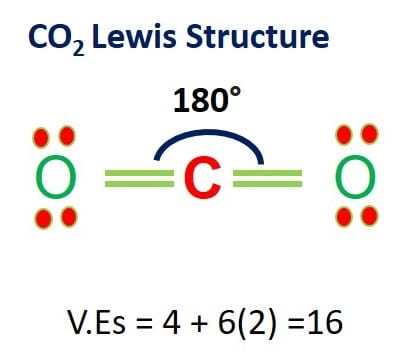
Lewis Structure of CO2
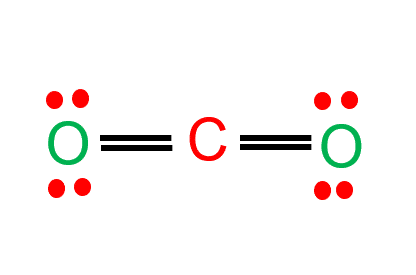
In the CO2 Lewis structure, there are two oxygen atoms and one carbon atom.
The least electronegative carbon atom is in the central position.
Oxygen forms sigma bonds with the central carbon atom to complete their octet.
As a result, there are no lone pairs of electrons, but bonding pairs of electrons also repel each other.
Due to these repulsive forces between the valence shell electron pairs, the molecule has a linear shape.
Why is CO2 nonpolar while CO is polar?
CO (Carbon monoxide) is a polar molecule because of the electronegativity difference between C(2.55) and O(3.44) atoms. Both atoms have unequal charge distribution, and therefore the CO bond has a net dipole moment making it a polar molecule.
On the other hand, CO2 is nonpolar because it has a linear, symmetrical structure.
The 2 oxygen atoms have equal electronegativity, pulling the electron density from carbon at an angle of 180 degrees from either direction. The two equal dipoles cancel out the effect of each other as they are pointed opposite to one another. That’s why CO2 has a zero dipole moment.
Since there’s no unequal sharing of valence electrons in the case of carbon dioxide, it is nonpolar.
Summary
- Carbon monoxide molecules consist of a carbon atom covalently double bonded to the oxygen atom.
- In the CO molecule, C-O length is 112.8 pm
- CO is a toxic gas.
- Each carbon and oxygen atom contains one lone pair of electrons.
- The molar mass of CO is 28.01 g/mol.
Related Links
N2O Lewis Structure
HCN Lewis Structure
SiO2 Lewis Structure
SO2 Lewis structure
BF3 Lewis structure| Molecular geometry, Hybridization
Frequently Asked Questions (FAQs)
The following are some of the frequently asked questions. If you have any questions related to this blog, please feel free to comment.
1. Is Carbon monoxide acidic or basic?
Carbon monoxide is a neutral gas since it does not show basic and acidic properties when it reacts with water
2. Explain CO dot structure in simple words
The overall carbon to oxygen atom ratio in a CO dot structure is 1:1. By sharing three valence electrons, carbon forms three tipple bonds with oxygen. Its octet is now complete. Similarly, to complete its octet, oxygen shares three valence electrons with carbon. Oxygen also possesses a lone pair of electrons.
3. Does CO have lone pairs?
There are two lone pairs in CO.
The lone pair of electrons are unshared valence electrons.
They are also called nonbonding pairs.
Lone pair of electrons are found in the outermost electron shell of atoms.
They can be identified by using a Lewis structure.
4. How to draw the dot structure of oxygen?
In the O2 Lewis structure, there is a double bond between two oxygen atoms.
Oxygen is a nonpolar diatomic molecule with a 180-degree bond angle.
Both oxygen atoms in the molecule have the same electronegativity value, and both atoms share identical ratios of bound shared electrons, making the O2 molecule nonpolar in nature.
5. How does CH4 produce carbon monoxide?
Methane (CH4) combines with steam under 3–25 bar pressure (1 bar = 14.5 psi) in the presence of a catalyst to produce hydrogen, carbon monoxide, and a tiny quantity of carbon dioxide in steam-methane reforming. Steam reforming is endothermic, which means that heat is required for the reaction to continue.
6. How many sigma bonds and pi bonds are in CO?
CO has one sigma bond and two pi bonds.
7. What is CLF3 molecular geometry?
ClF3 has a T-shaped molecular geometry and a trigonal bipyramidal electron geometry. According to the ClF3 Lewis structure, this molecule has two lone pairs and three bound pairs, according to the ClF3 Lewis structure. ClF3 is a polar compound.
More Links
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023