Carbon dioxide is a pure substance. A pure substance must be composed of just one type of molecule, or it may be an anionic or metallic solid composed exclusively of the atoms in the empirical formula, although not always of the same type of atom.
Carbon dioxide is a gas with a set composition that retains its identity even when subjected to minor changes in physical circumstances such as temperature and pressure and needs chemical modifications to transform into other substances.
On the other hand, a mixture does not have a definite makeup. Air is an example since it can include more or less of any of the existing gases, such as different amounts of water vapor.
Physical activities such as fractional liquification can frequently separate a mixture into its constituents (to separate nitrogen, carbon dioxide, oxygen, argon, and water vapor from the air).
- In the atmosphere, carbon dioxide is a greenhouse gas.
- Carbon dioxide accounts for less than 1% of the atmosphere, although it is a significant greenhouse gas.
- This implies that its molecules absorb radiation in the atmosphere, keeping the Earth warmer than it would otherwise be.
- Carbon dioxide is used to make beverages fizzy and to keep foods fresh.
Table of Contents
CO2 Molecular Geometry
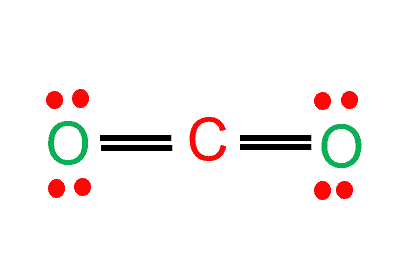
In CO2 molecular geometry, carbon makes a double bond with each of the two oxygen atoms, resulting in a tiny symmetrical, linear molecule of CO2 that is volatile and reasonably reactive.
Because oxygen atoms make sigma bonds with the central carbon atom to complete their octet, CO2 has a linear molecule shape. As a result, lone pairs of electrons are absent, yet bonded pairs of electrons repel one another. Due to these repulsive forces between the valence shell electron pairs, the molecule acquires a linear shape.
Summary
The answer to the question “Is carbon dioxide a pure substance?” is yes.
Pure substances do not need to be mixed and cannot be broken down without the use of sophisticated methods. Carbon dioxide is a pure substance as it maintains its identity even when subjected to small changes in physical conditions such as temperature and pressure and requires a chemical change to become other substances.
Related Topics
Molar Mass of Acetic Acid| Easy-Explanation
Concentration Gradient Definition
Sodium Phosphate – Formula, Structure, Types, and Uses
Sulfurous Acid| Formula & Lewis Structure
Valence Electrons in Nitrogen
Chemical Reactions Examples
Frequently Asked Questions
1. What are greenhouse gases?
Greenhouse gases contribute to the greenhouse effect by trapping heat in them. There are lots of gases that make up the earth’s atmosphere. Some of these gases are Carbon dioxide, methane, and Nitrous oxide. These three gases are called greenhouse gases.
2. Is carbon dioxide a pure substance?
Carbon dioxide is a fixed-composition gas that retains its identity even when subjected to minor physical changes like temperature and pressure.
3. What factors increase carbon dioxide in the atmosphere?
Processes that increase CO2 levels in the atmosphere include:
In automobiles, gasoline and diesel are burned.
Living creatures’ breathing
4. How we can decrease carbon dioxide in the atmosphere?
Photosynthesis is the process by which plants convert carbon dioxide and water into their own nourishment, glucose.
5. How To Reduce Carbon Footprint?
A few steps that could be taken in our daily life to reduce carbon footprint are as follows:
- Switch off the light when you leave a room.
- When possible, walk and use public transport.
- Recycle old electronics.
- Use eco-friendly recyclable alternatives to plastic bags.
- Plant more trees.
- Use water judiciously and go in for water harvesting.
- Compost your food waste.
- Unplug your electronic devices, when not in use.
- Take the stairs as often as possible.
- Reduce paper consumption.
6. Is Air a Homogeneous Mixture?
Air is a homogenous mixture of many gases. The atmosphere’s air is made up of nitrogen, oxygen (which is necessary for animal and human life), carbon dioxide, water vapor, and trace amounts of other elements (argon, neon, etc.). Higher elevations have more ozone, helium, and hydrogen in the air.
7. What is a Carbon footprint?
According to the World Health Organization, a carbon footprint is an assessment of the human impact on the earth’s natural greenhouse. Human activities such as the burning of fossil fuels, deforestation, and land-use changes are the primary sources of carbon emissions, which result in an increase in greenhouse gas concentrations in the atmosphere. To know more, check out the article “How to Reduce Carbon footprint.”
More Interesting Topics
Silicon Carbide – An Overview
SO2 Polar or Nonpolar
N2o Molecular Geometry
CH4 Polarity
Sigma Bond- Definition & Explanation
Is water a Pure Substance?| Answer
Delocalized pi bond| Definition
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023



