Water is a polar molecule.
A water molecule comprises two hydrogen atoms that are bonded to one oxygen atom. The large oxygen atom attracts electrons more strongly than the small hydrogen atoms. Because the oxygen atom has a stronger pull on the negative bonding electrons, it has a slightly negative charge, while the hydrogen atom has a positive charge. This unequal distribution of electrons is referred to as a polar bond or dipole. This causes the hydrogen atoms in one water molecule to bond to the oxygen atoms in another water molecule. This attraction is referred to as hydrogen bonding. Water can change from a liquid to a solid or a gas and back again, but its molecular structure remains constant. Keep on reading to find out more about the question “Why is water a polar molecule?”.
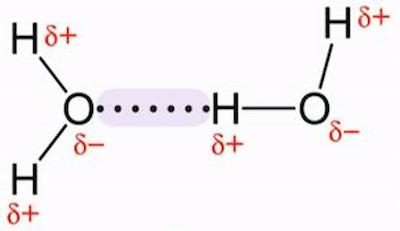
Table of Contents
Hydrogen Bond
Hydrogen atoms in water molecules form bonds with neighboring hydrogen and oxygen atoms in other water molecules. This causes the attraction of individual water molecules, resulting in the formation of a bond known as a hydrogen bond.
The polarity of a water molecule
Water (H2O) is a polar covalent molecule, as is hydrogen fluoride (HF). A water diagram (see Fig.) shows that the two hydrogen atoms are not evenly distributed around the oxygen atom. Because of the unequal distribution of electrons among the atoms and the asymmetrical shape of the molecule, a water molecule has two poles: a positive charge on the hydrogen pole (side) and a negative charge on the oxygen pole (side). The water molecule is said to be electrically polar.
Why water is a universal solvent?
Water is known as a “universal solvent” because it can dissolve more compounds than any other liquid. This feature of water is essential to all living things on the planet. It means that water transports valuable chemicals, minerals, and nutrients wherever it goes, whether through the air, the earth, or our bodies.
Water molecules are made up of polar oxygen and hydrogen atoms, with one side (hydrogen) having a positive electrical charge and the other side (oxygen) having a negative charge. This allows the water molecule to be attracted to a wide range of other molecules.
Summary
- The answer to the question “Why is water a polar molecule?” The reason is simple; water is a polar molecule because the oxygen atom has a stronger pull on the negative bonding electrons. That’s why oxygen has a slightly negative charge, while the hydrogen atom has a positive charge.
- A water molecule has two poles: a positive charge on the hydrogen pole (side) and a negative charge on the oxygen pole (side).
- Water is a universal solvent due to its polar nature.
- Water molecules’ hydrogen atoms form bonds with neighboring hydrogen and oxygen atoms in other water molecules. This causes individual water molecules to attract, resulting in the formation of a hydrogen bond.
Related Links
What Temp Does Water Freeze?| Science
Boiling of Water| Phenomenon and Factors Affecting
Surface Tension of Water
How Much is a Liter of Water?
Physical and Chemical Properties of Water
Frequently Asked Questions
1. Why does oil float on water?
Oil floats on the water It is less dense than water because its density is lower than that of water. Density in liquids is defined as the amount of mass that may be filled into a cubic meter of volume. Water has a density of roughly 1000 kg/cubic meter, while oil has a density ranging from 800 to 960 kg/cubic meter.
2. What is heavy water?
Heavy water (D2O), also known as deuterium, is made up of deuterium, a hydrogen isotope with twice the mass of ordinary hydrogen, and oxygen. Heavy water is used in nuclear power plants as a neutron moderator.
3. How many cups in a gallon?
A US liquid gallon is equal to 16 cups, and a US dry gallon is equal to 18.61 cups. In the United States, one cup equals half a pint (236.6 ml).
To get a more detailed answer, click “how many cups in a gallon.”
4. What is the kinematic viscosity of water?
The kinematic viscosity of water at 20 °C is about 1 cSt.
The physical unit for kinematic viscosity is the stokes (St), named after George Stokes. It is sometimes expressed in terms of centistokes (cS or cSt); 1 stokes = 100 centistokes = 1 cm2 s−1 = 0.0001 m2 s−1.
5. What is the Weight of Water?
Commonly used weight of water relations are given below:
1cm³ of water = 1 ml = 1 g.
1 L of water = 1000 ml = 1 kg = 1000 g.
1 US gallon of water equals 3.785 liters or 3.785 kg.
1 UK gallon of water = 4.546 liters = 4.546 Kg.
6. static pressure?
“Static pressure,” also called “ambient pressure,” is the pressure on the surface of an object at rest or in uniform linear motion. Static pressure is measured in millimeters of mercury (mmHg), kilograms per square meter (kg/m2), or pounds per square meter (Pa).
7. Liquid oxygen?
Liquid oxygen is a liquified form of oxygen gas that is utilized as an oxidant for liquid fuels in missile and rocket propellant systems. It is a pale blue liquid that is extremely cold. Although it is not flammable, it is a powerful oxidant.
8. Why water is a polar molecule?
Water is an example of a polar molecule.
A water molecule is made up of two hydrogen atoms linked to one oxygen atom. The big oxygen atom has a stronger attraction to electrons than the tiny hydrogen atoms.
9. Does water cause weathering or erosion?
Water causes weathering. After a rock has been broken down, a process known as erosion carries the rock and mineral fragments away. Check the full article “weathering vs erosion“.
10. Is water a pure substance?
Water is a pure substance, a hydrogen-oxygen compound. Although water is the most abundant substance on the planet, it is rarely found in its pure form in nature.
Please see the full article “Is water a pure substance?”.
11. What is the viscosity of water?
The viscosity of water at 20 degrees Celsius is 0.01 poise r 0.001 Pa.s (Pascal seconds).
More Interesting Topics
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023



