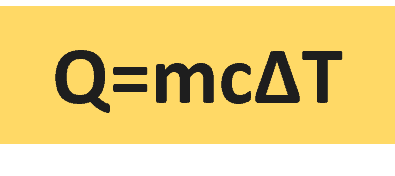The amount of heat required to raise the temperature of one kilogram mass of a substance by one Kelvin is referred to as specific heat (cp). The specific heat of water is 4182 J/kg°C which is higher than any other common substance. As a result, water plays a very important role in temperature regulation.

Table of Contents
Simple Words Explanation
“Specific heat” is the quantity of heat that must be added or taken away from a unit mass of a substance to change its temperature by 1 °C. It is an intensive property. In simple terms, it takes different amounts of heat to raise the same number of different substances through the same temperature range.
Its formula is Q/m ΔT = cp, where Q refers to the amount of heat supplied to the specimen and ΔT is the rise in temperature.
The value of cp depends on the nature of the substance.
Specific Heat Formula
Consider the temperature of a body of mass “m” changing by ΔT. The amount of heat (Q) absorbed or released by a body is proportional to its mass and change in temperature.
Q ∝ mass of the body (m) ………(1)
Q ∝ change in temperature (ΔT) ………(2)
Combining equation 1 and equation 2
Q ∝ mΔT ⇒ Q = cp.m.ΔT
Where cp is the constant of proportionality, it depends on the nature of the substance.
The formula shows that the transfer of heat to an object depends upon two factors:
- The change in temperature
- The mass of the system
For the same temperature change in twice as much mass, you need to add two times the heat. Heating capacity changes with temperature and pressure, so cp is often measured at the same temperature and pressure, like 25 degrees Celsius.
Key Points
- The specific heat of water is 4182 J/kg°C. This means that 1 kg of water takes 4182 joules of heat to raise its temperature by 1 degree Celsius.
- Water has an extremely high cp capacity, which makes it good for temperature regulation.
- It is the capacity of a substance that depends on the nature of the material.
- It is an intensive property that depends only on the type and phase of a substance.
- As per the mathematical formula, constant (cp) = ΔQ if the temperature change of 1 kg mass is 1K.
- Specific heat does not depend upon the size of the object.
Specific Heat of Water
The amount of heat required to raise the temperature of 1 gram of a substance by 1 degree Celsius.
The specific heat of water is 4182 J/kg°C which is higher than any other common substance. As a result, water plays a very important role in temperature regulation.
Water has a high specific heat, which means it takes more energy to heat it than other substances. This is why water is used in industries and as a coolant in cars. Water’s high specific heat helps regulate the rate at which air temperature changes, which is why seasonal temperature changes are gradual, especially near the oceans.
Specific Heat Formula- Sample Problem
A container has 2.5 liters of water at 20°C. What is the cp required to boil the water? The density of water is 1000 kgm-3 and the specific heat of water is 42000 J Kg-1 k-1.
The volume of water is 2.5 liters and the mass of water is 2.5 kg.
Initial temperature T1 = 20 °C
Final temperature T2 = 100 °C
ΔT = T2-T1
= 100°C-20°C = 80°C or 80K
Q = cp.m.ΔT = 42000 x 2.5 x 80 = 840000J = 840 kJ
Summary
- Specific heat refers to the exact amount of heat needed to make one unit of mass of a substance one degree warmer.
- The specific heat formula is Q = m.ΔT.cp
- The SI unit of cp is joules per kilogram per kelvin.
- The specific heat of water is 4182 J/kg°C.
Related Topics
Mechanical Energy Formula & Examples
Silicon Carbide – An Overview
What Is Auxiliary Heat| 5 Easy Key Points
Heat Flux-An Overview
Thermal Energy Equation- Simple Overview
Chemical Formula for Water
Soft Water vs. Hard Water
What is the Specific Heat of Air?
Frequently Asked Question
Some of the frequently asked questions are given below
1. What is heat flux?
The quantity of heat transferred per unit area per unit time to or from a surface is referred to as heat flux.
2. What is a water molecule and how many hydrogen atoms are in a molecule of water?
Water is a substance that exists in gaseous, liquid, or solid phases and is made up of the chemical elements hydrogen and oxygen. It is one of the most abundant and necessary chemicals. At room temperature, it is a tasteless and odorless liquid with the critical capacity to dissolve many other compounds. Water’s capacity as a solvent is critical to living creatures.
A water molecule consists of two hydrogen atoms attached to the sides of a single oxygen atom.
3. What is thermite welding?
Thermite welding is a type of welding that involves reducing metal oxides with aluminum powder and releasing a large quantity of heat. A thermite reaction is used to repair railway rails or broken machine components.
4. Difference between heat capacity and specific heat capacity?
Specific heat capacity is the heat required to raise the temperature of a substance by 1 degree Celsius. Heat capacity is the ratio of heat absorbed by a material to the temperature change.
5. Specific heat formula?
Specific heat formula is the amount of heat supplied to the specimen, divided by the resulting temperature increase. cp is related to the unit mass of the specimen. The mathematical form of the formula is Q/m ΔT = cp where Q refers to the amount of heat supplied to the specimen and ΔT is the rise in temperature.
6. Weight of water in kg?
At 25°C (77°F or 298.15K), the weight of water is1 gram per cubic centimeter, or 1,000 kilograms per cubic meter. This means that the density of water is 1 000 kg/m3. Weight per cubic foot: 62.4 pounds per cubic foot [lb/ft3], or 0.58 ounces per cubic inch [oz/inch3].
7. What is the importance of water in weathering?
Water is extremely important in chemical weathering in three ways. First, it reacts with carbon dioxide in the soil to form carbonic acid, a weak acid. Finally, hydrolysis can be used by water to break up minerals. This process decomposes the most common mineral group, silicates.
8. Density of water in lbft3?
Density is a physical property that expresses the mass per unit volume. In the case of liquid water, the density of water is close to 1000 kg/m3, though it varies slightly depending on temperature. The density of water lb/ft3 is 62.428 lb/ft3.
9. Thermal conductivity of water?
Thermal conductivity is a property of materials that indicates their ability to conduct heat. The thermal conductivity of water is 0.591 W/mK. It is frequently denoted by the letter ‘k’ or, less frequently, the letter “lambda.” Thermal resistivity is the reciprocal of thermal conductivity.
10. Is water a pure substance?
Pure water is also known as distilled water or deionized water. In distilled water, evaporation removes all of the dissolved substances. As it evaporates, water either distills or leaves the salt behind. Pure water is collected and condensed to produce distilled water.
11. What is portable water?
Water fit for human consumption is defined as potable water (i.e., water that can be used for drinking or cooking). The term implies that the water is both safe and drinkable.
12. What is the kinematic viscosity of water?
The kinematic viscosity of water at 20 °C is about 1 cSt.
The physical unit for kinematic viscosity is the Stokes (St), named after George Stokes. It is sometimes expressed in terms of centistokes (cS or cSt); 1 stokes = 100 centistokes = 1 cm2 s−1 = 0.0001 m2 s−1.
13. How many cups in a gallon?
A US liquid gallon is equal to 16 cups, and a US dry gallon is equal to 18.61 cups. In the United States, one cup equals half a pint (236.6 ml).
To get a more detailed answer, click “how many cups in a gallon.”
14. What is the melting point of water?
The melting point of pure water ice at 1 atmosphere of pressure is very close to 0 °C, which is 32 °F or 273.15 K.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023