Chlorine trifluoride (ClF3) is a colorless, poisonous, corrosive, and extremely reactive gas. ClF3 is a polar molecule due to its asymmetrical structure and the existence of two lone pair electrons, which results in an uneven distribution of charge, making it polar in nature. Keep reading to learn more about clF3 polarity, bond angle, and molecular shape.
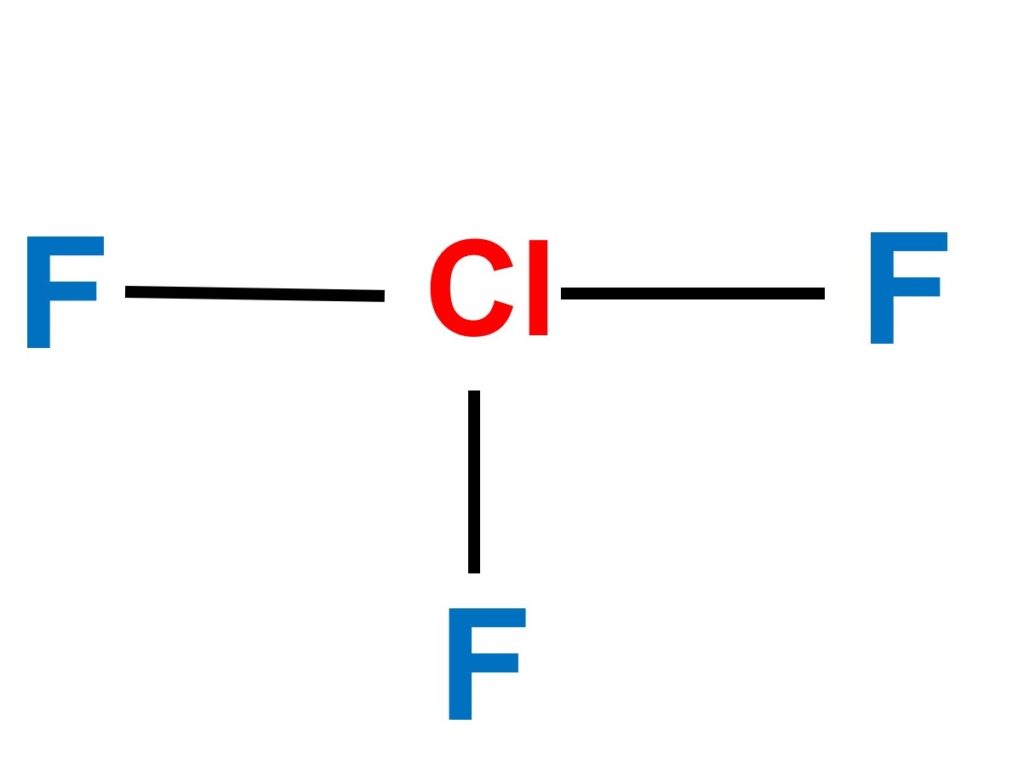
Table of Contents
Chlorine Trifluoride (ClF3)
ClF3 is formed by a cation chloride central which is bound to 3 anions fluoride.
In chlorine trifluoride, there are five zones of high electron density surrounding the central chlorine atom (3 bonds and 2 lone pairs).
The molecular geometry of ClF3 is stated to be T-shaped. It takes on this form due to the existence of two lone pairs that take up equatorial locations and have higher repulsions. Around the core atom, there is also an asymmetric charge distribution.
ClF3 is produced by heating a combination of fluorine and chlorine at around 280 °C in a vessel constructed of nickel, Monel metal (a corrosion-resistant alloy of nickel and copper-containing approximately 67 percent nickel and 30 percent copper), or Kel-F (poly(chlorotrifluoroethylene).
ClF3 is an extremely toxic and corrosive gas that can be lethal if breathed in.
It can also cause severe harm to the skin and eyes, making it very poisonous.
It has the potential to cause unexpected explosions.
| Name of molecule | Chlorine Trifluoride (ClF3) |
| bond angles of clf3 | less than 90 degrees |
| clf3 structure | Trigonal bipyramidal geometry |
| clf3 polar or nonpolar | polar molecule |
| clf3 valence electrons | 28 |
| clf3 steric number | 5 |
ClF3 Hybridization
The central chlorine atom in ClF3 is roughly sp2 hybridized. This indicates that three sp2 orbitals will emanate from the chlorine, forming an equatorial plane and either containing a lone pair of electrons or bonding to a ligand. We also have one unhybridized p-orbital perpendicular to the equatorial plane in the case of sp2 hybridization.
ClF3 Polar or Nonpolar
ClF3 is a polar molecule due to its asymmetrical structure and the presence of two lone pair electrons, which results in an unequal distribution of charge and so makes it polar.
ClF3 Molecular Geometry
ClF3 has a T-shaped molecular geometry and trigonal bipyramidal electron geometry. This molecule has two lone pairs and three bound pairs, according to the ClF3 Lewis structure. ClF3 is a polar compound.
Related Links
sicl4 polar or nonpolar
Perchloric acid| Is It Super Acid?
Is HCl Polar or Nonpolar?
Is Nh3 Polar?
SiO2 Lewis Structure| Step By Step Construction
Frequently Asked Questions
1. Is SiCl4 polar or nonpolar?
SiCl4 (silicon tetrachloride) is a nonpolar molecule. Because the four chemical bonds between silicon and chlorine are uniformly distributed, SiCl4 is non-polar. A polar covalent bond is a type of covalent link that is intermediate between pure covalent bonds and ionic bonds. When the difference in electronegativity between the anion and the cation is between 0.4 and 1.7, such bonds occur.
Check the full article “Is SiCl4 polar or nonpolar?”.
2. Is BF3 polar or nonpolar?
BF3 is a non-polar compound. In BF3, the central boron atom has sp2 hybridized orbitals, resulting in an unfilled p orbital on the Bron atom and trigonal planar molecular geometry. Because the boron-fluorine bonds are all 120 degrees apart, any net dipole in that plane is canceled out. Even if each B-F bond is polar, the net dipole moment is zero because adding the bond vectors cancels everything out.
Check the full article “Is BF3 polar or nonpolar?”.
More Interesting Topics
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023



