A polar covalent bond is one in which the atoms are attracted to electrons in different amounts, resulting in unequal sharing. In a polar covalent bond, the electronegativity difference between the atoms is between 0.4 and 1.7.

Table of Contents
Covalent Bond Definition
A covalent bond between two atoms is formed when the electrons from each participating atom are shared equally. A shared pair, or bonding pair, refers to the pair of electrons involved in this form of bonding. The atoms acquire stability in their outer shell through the sharing of bonding pairs, analogous to noble gas atoms.
Types of Covalent Bonds
- Single Covalent Bond
- Double Covalent Bond
- Triple Covalent Bond
Polar Covalent Bond Definition
Polar covalent bonds are a kind of covalent bond that lies between pure covalent bonds and ionic bonds. Such bonds are formed when the difference in electronegativity between the anion and the cation is between 0.4 and 1.7.
Polar vs. Nonpolar
Polar molecules are formed when the electronegativity of the bonded atoms differs. When electrons are shared equally between atoms in a diatomic molecule or when polar bonds in a larger molecule cancel each other out, nonpolar molecules form.
Examples of polar molecules include water, ammonia, sulfur dioxide, hydrogen sulfide, and ethanol.
Examples of nonpolar molecules include helium, neon, argon, krypton, and Xenon.
Please refer to the full article“Why is water a polar molecule?”.
What is polarity?
The distribution of electrical charge over the atoms joined by the bond causes polarity.
Specifically, it is found that bonds between atoms of different elements are electrically inequivalent. In hydrogen chloride, for instance, the H atom is slightly positively charged whereas the Cl atom is slightly negatively charged.
Partial charges are the little electrical charges that exist on different atoms, and the existence of partial charges indicates the presence of a polar bond.
Examples of polar molecules
Some examples of polar molecules are Water (H2O), Ethanol, Ammonia, and SO2 (Sulfur Dioxide).
HCL Polar or Nonpolar
HCl (hydrochloric acid) is a polar molecule. Since chlorine is more electronegative than hydrogen, it pulls the bonded electron pair closer to it and obtains a partial negative charge, whereas hydrogen gains a partial positive charge. HCl has a dipole moment of 1.03 D.
CO2 Polar or Nonpolar
Carbon dioxide (CO2) is nonpolar because of its linear and symmetrical structure. The two oxygen atoms from either direction of the carbon atom pull the electrons equally from both sides. Due to unequal sharing of valence electrons, CO2 is nonpolar in nature.
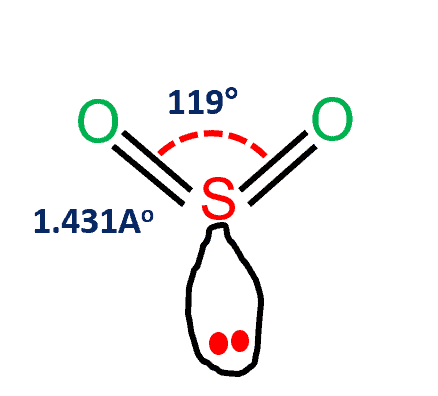
SO2 Polar or Nonpolar
The gas sulfur dioxide (SO2) has a polar character. It’s a polar molecule because of the electronegativity difference between the sulfur (2.58) and oxygen (3.44) atoms. SO2 has a bent form due to the existence of unbonded electrons on the sulfur and oxygen atoms.
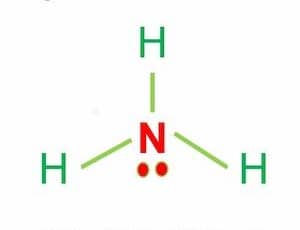
The polarity of ammonia (NH3)
NH3 is a polar molecule because it has three nitrogen-hydrogen bond dipoles that do not cancel out.
The electronegativity difference between N (3.04) and H (2.2) causes the polarity of the NH3 molecule.
This mismatch in electronegativity results in three dipole moments in one direction.
As a result, ammonia has a net dipole moment, making it a polar molecule.
In addition, the NH3 Lewis structure shows that there is a lone pair of electrons present in nitrogen.
This exerts an outward force on the bond due to which the shape of NH3 becomes unsymmetrical.
Polarity of H2S
H2S is a slightly polar molecule because of the minor difference in electronegativity values of hydrogen (2.2) and sulfur (2.58) atoms. Furthermore, the existence of two lone pairs on the opposite side of the two hydrogen atoms makes the molecule more polar and causes the H2S molecule to have a bent shape geometrical structure.
Frequently Asked Questions
1. Polar covalent bond?
Polar molecules form when the electronegativity of the connected atoms changes. When electrons are evenly divided between atoms in a diatomic molecule or when polar molecules in a larger molecule cancel each other out, nonpolar molecules arise.
2. What is a hydrogen bond?
A hydrogen bond (H-bond) is a dipole-dipole interaction. It is not an actual chemical bond.
It develops when a hydrogen atom is bonded to a strongly electronegative atom.
(fluorine, nitrogen, or oxygen ) exists in the vicinity of another electronegative atom with a lone pair of electrons.
This type of bond is weaker than an ionic or covalent bond, but it is stronger than van der Waals forces.
3. What is the electronegativity difference?
Electronegativity refers to an atom’s tendency to attract electrons to itself in a chemical bond.
The greater the difference between atom electronegativity values, the more polar the chemical bond formed between them.
4. Define dipole moments
A dipole moment is simply a measurement of the net polarity of a molecule.
When polar bonds are irregularly scattered around the core of a molecule, the charge distribution throughout the entire molecule is uneven, resulting in a polar molecule.
Ammonia is an example of a polar molecule (NH3).
The electrons they share are drawn to nitrogen because nitrogen attracts electrons more strongly than hydrogen.
5. Ch4 polar or nonpolar?
Methane (CH4) is a nonpolar molecule.
CH4 has an asymmetric tetrahedral geometrical geometry with four identical C-H bonds. The electronegativity of carbon and hydrogen is 2.55 and 2.2, respectively, resulting in almost zero partial charges.
More Links
SO2 Ionic or Covalent?| Simple Explanation
Density of Oxygen
Ionic Compounds| Properties, and Examples
HCL Intermolecular Forces
What is the molar mass for sulfur dioxide?
Is MgCl2 Ionic or Covalent?
Charge of Ammonia (NH3)| Simple Steps
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023