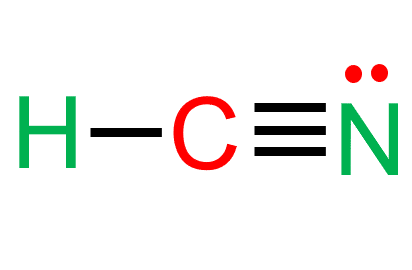A delocalized pi bond is a pi (π) bond is one in which electrons can move freely between more than two nuclei.
Localized electrons behave normally, and a localized lone pair stays close to one atom. Instead of staying with one atom, electrons in a delocalized pi bond visit two.

Table of Contents
Examples of the delocalized PI bond
The O3 molecule contains the π bonds between the atoms, which are delocalized on the oxygen atoms. Hydrogen cyanide (HCN) also contains the π bond between carbon and nitrogen, but the bond is localized as its hydrogen atom cannot accommodate the double or triple bond. For more details, check the links for O3 lewis structure and HCN lewis structure.
The electrons in the bond of a molecule like ethylene are confined to the area between the two carbon atoms. In buta-1,3-diene, however, the two orbitals can overlap, and the electrons are free to spread over all four carbon atoms. These electrons are said to be delocalized.
Daily Life Significance of Delocalized Pi Bond
| Aromatic compounds | Many aromatic compounds found in nature, such as the scent of flowers, spices, and perfumes, contain delocalized pi bonds. They are also used in synthetic materials like plastics, fibers, and dyes. |
| Color of organic compounds | Delocalized pi bonds in organic compounds, such as pigments and dyes, can cause them to have unique and vibrant colors. For example, the green color of chlorophyll, the red color of carotenoids, and the blue color of anthocyanins are all due to the presence of delocalized pi bonds. |
| Conductivity of polymers | Some polymers, such as conductive plastics, conductive rubbers, and conducting polymers, contain delocalized pi bonds that enable them to conduct electricity. |
| Drug development | The ability to predict and understand the properties of delocalized pi bonds is important in drug design and development, as many drugs have aromatic compounds that contain delocalized pi bonds. |
| Solar cells | Organic solar cells often contain conjugated systems with delocalized pi bonds that absorb sunlight and create electrical current. |
What is the Pi bond?
A pi bond (bond) is a bond produced by the overlap of p orbitals on neighboring atoms that is perpendicular to any sigma bond(s) between the same atoms. A pi bond is a covalent bond that is formed by lateral overlapping of the half-filled atomic orbitals of atoms.
Delocalized Electron
A delocalized electron is one that is not bonded to a single atom or covalent bond in an atom, ion, or molecule.
Delocalisation of an electron occurs when an atom’s valence electron leaves its particular shell and begins to move freely in the valence shells of its covalently bound molecule.
The extra energy produced by the ring orbitals of the delocalized electrons from the “pi” bonds that are spread out over the entire covalently bound molecule is referred to as delocalization energy. Because the electrons do not linger over one atom and continue to rotate, the molecule is more stable. It is also known as the stabilization energy since it helps to keep the entire molecule stable.
Examples
| Molecule or Compound | Explanation |
|---|---|
| Benzene | Benzene is a six-carbon aromatic compound that contains delocalized pi bonds. The pi electrons in benzene are spread out over all six carbon atoms in a hexagonal ring. |
| Ethene | Ethene is a two-carbon molecule with a double bond between the carbon atoms. The pi electrons in the double bond are delocalized over both carbon atoms and the bond is shorter and stronger than a localized pi bond. |
| Acetylene | Acetylene is a two-carbon molecule with a triple bond between the carbon atoms. The two pi bonds in the triple bond are delocalized over both carbon atoms and the bond is shorter and stronger than a localized pi bond. |
| Nitrogen gas | Nitrogen gas (N2) is a diatomic molecule that contains a delocalized pi bond. The two nitrogen atoms share a triple bond, which includes a pi bond formed by the delocalization of the two pi electrons. |
| DNA bases | The nitrogenous bases that make up the rungs of the DNA ladder contain delocalized pi bonds. For example, the base adenine contains a delocalized pi bond that spans several atoms in the ring structure. |
Summary
- A delocalized pi bond is a type of chemical bond where the pi electrons are spread out over more than two atoms, rather than being localized between just two adjacent atoms.
- In a delocalized pi bond, the pi electrons are spread out over a larger region, involving more than two atoms, and can be found in orbitals that span multiple atoms.
- Molecules or compounds that contain conjugated systems, such as benzene or other aromatic compounds, typically have delocalized pi bonds.
- Resonance is a phenomenon that occurs when a molecule or compound has delocalized pi bonds, and there are multiple ways to draw Lewis structures that represent the molecule or compound.
- Resonance increases the stability of molecules or compounds with delocalized pi bonds, because it allows the pi electrons to be more spread out over a larger region, lowering the overall energy of the system.
- The length of a delocalized pi bond is inversely proportional to its strength. A shorter delocalized pi bond is stronger than a longer one.
Related Topics
| Bond Length Definition| Chemistry | Polar Covalent Bond-Definition and Examples |
| Single Bond | Covalent Bond Simple Explanation |
Frequently Asked Questions
Some of the frequently asked questions are given below:
1. How many sigma bonds are in a triple bond?
One sigma bond and two pi bonds make up a triple bond.
2 How to count sigma and pi bonds?
The following rules are all you need to know:
A single bond is equal to one sigma bond.
A double bond is made up of one sigma and one pi bond.
1 sigma and 2 pi bonds equal a triple bond.
3. What is a sigma bond example?
The triple bond between the two nitrogen atoms in the molecule nitrogen (N2), for example, consists of a sigma bond and two pi bonds.
4. Heat flux?
The rate of thermal energy flow per unit surface area of a heat transfer surface, such as in a heat exchanger, is known as heat flux.
5. Electric field units?
In the meter-kilogram-second and SI systems, electric field units are newtons per coulomb, which are equivalent to volts per meter.
6. Paramagnetic materials?
Paramagnetic materials are metals that are weakly attracted to magnets. Aluminum, gold, and copper are among them.
7. Is chlorine a metal?
Chlorine is nonmetal since it lacks metal-like properties such as electrical conductivity, flexibility, and strength.
8. Calcium electron configuration?
Calcium is a silvery-white, soft metal that tarnishes rapidly in the air and reacts with water.
calcium electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2
9. How many electrons does helium have?
In a single helium atom, there are 2 protons, 2 electrons, and 2 neutrons. Helium is the second element in the periodic table. Check the full article “How many electrons does helium have?”.
10. Valence electrons in nitrogen?
Nitrogen has 5 valence electrons. Nitrogen (N) is a non-metallic element in Periodic Group 15 [Va]. It is a colorless, odorless, and tasteless gas that is the most abundant element in the Earth’s atmosphere and is found in all living things.
Exam Related Questions
| # | Exam Questions | Answer |
|---|---|---|
| 1 | What is a delocalized pi bond? | A delocalized pi bond is a type of chemical bond where the pi electrons are spread out over more than two atoms, rather than being localized between just two adjacent atoms. |
| 2 | How is a delocalized pi bond different from a localized pi bond? | In a localized pi bond, the pi electrons are shared only between two adjacent atoms. In a delocalized pi bond, the pi electrons are spread out over a larger region, involving more than two atoms. |
| 3 | What types of molecules or compounds typically have delocalized pi bonds? | Molecules or compounds that contain conjugated systems, such as benzene or other aromatic compounds, typically have delocalized pi bonds. |
| 4 | What is resonance in the context of delocalized pi bonds? | Resonance is a phenomenon that occurs when a molecule or compound has delocalized pi bonds, and there are multiple ways to draw Lewis structures that represent the molecule or compound. |
| 5 | How does resonance affect the stability of molecules or compounds with delocalized pi bonds? | Resonance increases the stability of molecules or compounds with delocalized pi bonds, because it allows the pi electrons to be more spread out over a larger region, lowering the overall energy of the system. |
| 6 | What is the relationship between the length of a delocalized pi bond and its strength? | The length of a delocalized pi bond is inversely proportional to its strength. A shorter delocalized pi bond is stronger than a longer one. |
More Interesting Topics
Co2 Polar Or Nonpolar
SO2 Polar Or Nonpolar
CH4 Lewis Structure and Molecular Geometry
SO2 Lewis Structure: 4 Simple Steps
Single Bond
Hydrogen Bond|Definition & Easy Explanation
Is Titanium Magnetic?
CH4 Polarity
Sulfur Electron Configuration
HCN (Hydrogen Cyanide) Hybridization
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023