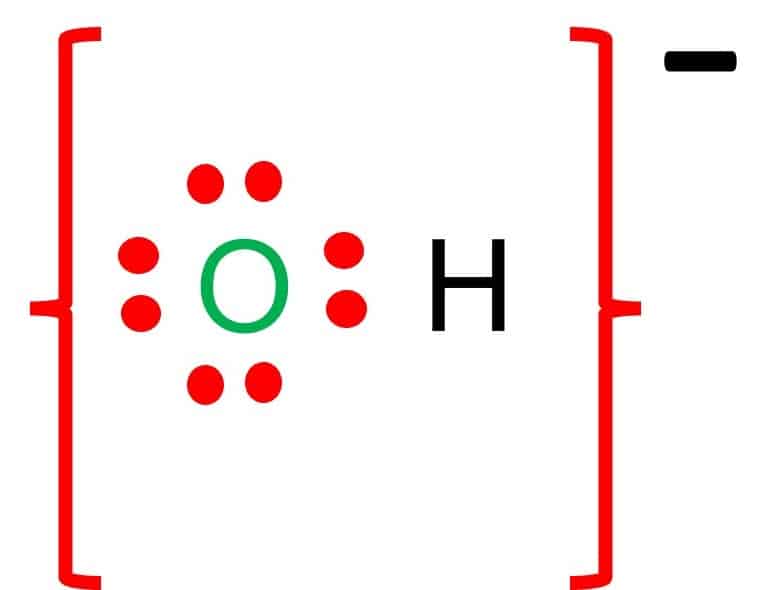
The hydrogen phosphate formula is [HPO4]2-. The molar mass of this compound is 95.97 g/mol. The ion is formed by the loss of two protons H+ from phosphoric acid H3PO4, which accounts for the ion’s charge of 2. Hydrogen phosphate, alternatively known as monohydrogen phosphate, is a naturally occurring inorganic ion with biological properties. It is used as a food additive in the industry.
![hydrogen phosphate formula structure and uses. its formula is [HPO4]-2](https://whatsinsight.org/wp-content/uploads/2022/01/hydrogen-phosphate.jpg)
Table of Contents
Hydrogen Phosphate Formula
Hydrogen phosphate, alternatively referred to as monohydrogen phosphate, is a naturally occurring inorganic ion that serves a biological purpose. It is a food additive in the industry. Its formula is [HPO4]2-.
Frequently Asked Questions
1. Is sodium phosphate soluble?
Sodium phosphate (Na3PO4) is soluble in water.
Furthermore, The carbonates, phosphates, borates, sulfates, chromates, and arsenates of all metals except sodium, potassium, and ammonium are insoluble in water but soluble in dilute acids.
2. Is sodium in sodium phosphate a cation or anion?
Sodium phosphate is an ionic compound composed of sodium cation and phosphate anion.
Cation is a positively charged ion, i.e. one that would be attracted to the cathode in electrolysis.
3. What is sodium phosphate formula and molar mass?
Sodium phosphate formula is Na3PO4
molar mass = (3 x mol mass of Na) + mol mass of P+ (4 x mol mass of Oxygen)
molar mass of Na3PO4 =3×23+1×31+4×16=164gmol−1
4. What are the sources of Phosphorus?
Phosphorus is naturally present in protein-rich foods such as meats, poultry, fish, nuts, beans, and dairy products (organic phosphorus). Phosphorus is found in grains, particularly whole grains. It can also be found in tiny levels in fruits and vegetables.
5. Sodium Tripolyphosphate?
The sodium salt of the polyphosphate Penta-anion, sodium tripolyphosphate, is an inorganic chemical having the formula Na5P3O10. It’s found in a variety of household and commercial goods, notably detergents.
6. Is MgCl2 Ionic or Covalent?
When the magnesium atom loses two electrons to create the Mg2+ ion and each chlorine receives one electron to form the Cl– ion, an ionic connection is formed between the magnesium and chlorine atoms.
Check “Is MgCl2 ionic or covalent?”.
7. What is hydrogen phosphate?
The formula of hydrogen phosphate is [HPO4]2-. The molar mass of this compound is 95.97 g/mol. The ion is formed by the loss of two protons H+ from phosphoric acid H3PO4, which accounts for the ion’s charge of 2.
8. What is a hydrogen ion?
The nucleus of a hydrogen atom separated from its electron is known as a hydrogen ion. The hydrogen nucleus is made up of a proton, which is a particle with a unit positive electric charge.
9. Is hydrogen sulfide poisonous?
Hydrogen sulfide (H2S) is made up of a single sulfur atom bonded to two hydrogen atoms. It is a highly poisonous, flammable gas with the odor of rotten eggs that are frequently produced by bacterial decomposition of organic matter in the absence of oxygen.
10. What is the electronegativity value of hydrogen?
The electronegativity of hydrogen is 2.2.
Electronegativity is used to determine whether an ionic or covalent connection will form between two atoms. It can also determine whether the molecule is polar or nonpolar.
More Interesting Topics
Sodium Hydrogen Sulfate
Hydrogen oxide-An Overview
Charge of Ammonia (NH3)| Simple Steps
Ideal Gas Law| Simple Overview
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023