A single bond is a chemical linkage consisting of one covalent bond between two atoms of a molecule, represented in chemical formulas by one line or two vertical dots, as C–H or C: H.
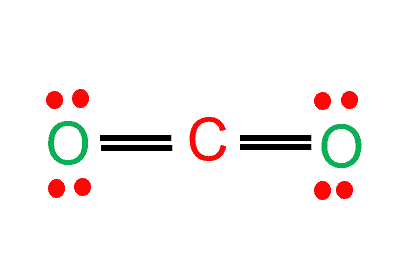
A single line indicates a single bond between two atoms (i.e., involving one electron pair), double lines (=) indicate a double bond between two atoms (i.e., involving two electron pairs), and triple lines (≡) represent a triple bond, as found, for example, in carbon monoxide (C≡O).
Table of Contents
Hydrogen Forms a Single Bond
The hydrogen atom form only one covalent bond to other atoms in the most stable neutral compounds.
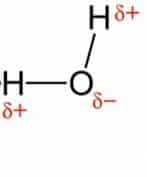
Water is a simple molecule made up of one oxygen atom linked to two hydrogen atoms through a single covalent bond.
The bonds are polar covalent due to the greater electronegativity of the oxygen atom (polar bonds).
For more details about the structure and isotopes of hydrogen, please check ” How many neutrons does hydrogen have”
What is Sigma Bond?
All single bonds are, in general, sigma bonds.
In chemistry, sigma bonds (bonds) are the strongest type of covalent chemical bond. They are formed when atomic orbitals collide head-on. The symbol for a sigma bond is σ
Sigma bonds are the strongest covalent connections due to the direct overlapping of the involved orbitals. The electrons involved in a bond are referred to as electrons.
Freqently Asked Questions
1. Is MgCl2 Ionic or Covalent?
When the magnesium atom loses two electrons to create the Mg2+ ion and each chlorine receives one electron to form the Cl– ion, an ionic connection is formed between the magnesium and chlorine atoms.
Check “Is MgCl2 ionic or covalent?”.
More Interesting Topics
SO2 Ionic or Covalent?
CH4 Lewis Structure & Molecular Geometry
Electromagnetic Force
O3 Lewis Structure | Step By Step Drawing
HCN Lewis Structure & Molecular Geometry
Hydrogen Bond| Definition & Easy Explanation
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023