Sulfur (sulphur in British English) is a chemical element with the symbol S and the atomic number 16. It is nonmetallic, abundant, and multivalent. Its atoms combine to produce cyclic octaatomic molecules. Sulfur is a beautiful yellow crystalline solid at room temperature. The sulfur electron configuration is 1s2 2s2 2p6 3s2 3p4 and is seen in the image below. Sulfur isotopes are 32S (95.02%), 33S (0.75%), 34S (4.21%), and 36S (0.02%).
The molar mass of sulfur is 32.065 u.
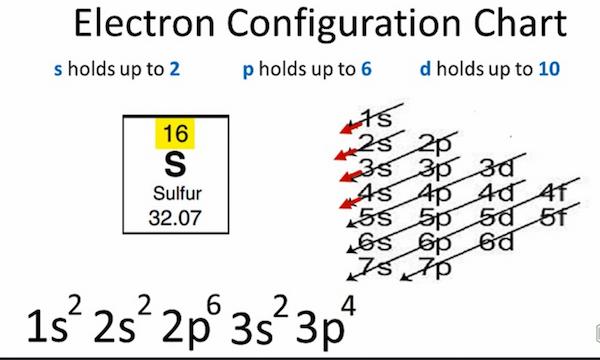
Table of Contents
Sulfur- Key Points
- Atomic number: 16
- Atomic weight: 32.066.
- Melting point: 388.36 K (115.21°C or 239.38°F)
- Boiling point: 717.75 K (444.60°C or 832.28°F)
- Density: 2.067 grams per cubic centimeter.
- Phase at room temperature: solid
- Element Classification: Non-metal
- Period number: 3
- Sulfur isotopes are 32S (95.02%), 33S (0.75%), 34S (4.21%), and 36S (0.02%).
- The molar mass of sulfur is 32.065 u.
Sulfur Dioxide
Sulfur Dioxide (American English), also known as Sulfur Dioxide (Commonwealth English), is a colorless, poisonous, inorganic gas with a noxious odor similar to nitric acid. Volcanic activity releases this gas naturally. It produces a mild acid solution when dissolved in water.
Sulfur dioxide is a major precursor of sulfuric acid and is present in small amounts in the environment.
What is sulfur used for?
Sulfur is used to vulcanize black rubber, as a fungicide, and in the production of black gunpowder. On the other hand, the majority of sulfur is used to make sulfuric acid, which is arguably the most significant chemical produced by western civilizations.
Sulfur Isotopes
Sulfur has four stable isotopes: 32S (95.02%), 33S (0.75%), 34S (4.21%), and 36S (0.02%).
Isotopes are different nuclear forms of the same element. For a given element, a constant number of protons but different numbers of neutrons in the nucleus correspond to different isotopes.
Sulfur Isotopes are mainly used in medical applications. S-33 is used for the production of the therapeutic radioisotope P-33. S-32 is used for the production of the radioisotope P-32 which is also used for therapeutic purposes. S-34 can be used for the production of the medical radioisotope Cl-34m and for S-35.
Summary
- Sulfur electronic configuration is 1s2 2s2 2p6 3s2 3p4
- Sulfur has six valence electrons, which implies that each atm has six electrons in its outermost shell.
- Sulfur is a beautiful yellow crystalline solid at room temperature.
- Sulfur is employed in the vulcanization of black rubber, as a fungicide, and in the manufacture of black gunpowder.
- Sulfur isotopes are 32S (95.02%), 33S (0.75%), 34S (4.21%), and 36S (0.02%).
- The molar mass of sulfur is 32.065 u.
Related Topics
SO2 Lewis Structure| 4 Simple Steps
SO2 Polar or Nonpolar
Is BF3 Polar or Nonpolar?
CH4 Lewis Structure & Molecular Geometry
Frequently Asked Questions
1. Is sulfur a metal?
Sulfur is a chemical element with an atomic number of 16 and the symbol S. It is a solid at ambient temperature and is classified as a nonmetal.
2. How many valence electrons does sulfur have?
Sulfur contains six valence electrons, which means it has six electrons in its outermost shell in each atom.
Each element’s number of valence electrons depends upon its location on the periodic table, though this only applies to neutral atoms.
3. Sulfur tetrafluoride?
Sulfur Tetrafluoride (SF4) is a colorless, corrosive gas that is used to make a variety of organofluorine chemicals.
SF4 is a hazardous chemical that is widely used in the chemical and pharmaceutical industries. The molecular geometry of SF4 is trigonal bipyramidal.
4. How many neutrons does sulfur have?
The atomic number of sulfur is 16, which implies it contains 16 protons. Sulfur is made up of 32 nucleons, including 16 protons and 16 neutrons.
5. is Sulfur malleable?
Sulfur is a nonmetal and therefore would not be malleable.
6. Sulfur oxidation number?
The oxidation number of sulfur depends on the compound it is in.
For instance,
In Perchloric acid (H₂SO₄), the oxidation number of S is +6.
In H₂SO₃, the oxidation number of S is +4.
In Na₂S₂O₃, the oxidation number of S is +2.
In S₈, the oxidation number of S is 0.
In Hydrogen sulfide (H₂S), the oxidation number of S is -2.
7. is Sulfur flammable?
Yes, sulfur is flammable. When sulfur is burnt, it produces sulfur dioxide, which is a gas. When the gas reacts with moisture on plants, an acid is formed, which can harm plant leaves. Inhaling the gas might be dangerous to one’s health.
8. What is sulfur electronic configuration?
Sulfur electronic configuration is 1s2 2s2 2p6 3s2 3p4
9. What’s the molar mass of Aluminum?
Aluminum molar mass is 26.982 g/mol.
10. How many electrons does oxygen have?
A single oxygen atom has eight protons, eight electrons, and eight neutrons.
Oxygen is a stable isotope of oxygen with a nucleus of 8 neutrons and 8 protons. Its mass is 15.99491461956 u. Check the full topic “How many electrons does oxygen have?”.
11. What is calcium’s electronic configuration?
Calcium electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2
12. How many electrons does helium have?
A single Helium atom has two protons, two electrons, and two neutrons. The second element in the periodic table is helium. Because s subshells may store up to two electrons, both electrons fit into the 1s subshell, resulting in the electron configuration for helium atoms being 1s2.
More Interesting Links
CO Lewis Structure & Molecular Geometry
Hydrogen Bond| Definition & Easy Explanation
H2S Lewis Structure & Molecular Geometry
Molar Mass of Methanol
What is the Molar Mass of Nitrogen?
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023



