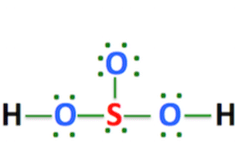
Acidic hydrogen is a hydrogen atom that has a tendency to forms a positive ion when the acid dissociates. Hydrogens that are directly linked to extremely electronegative atoms such as oxygen, sulphur, and halogens have high acidity.
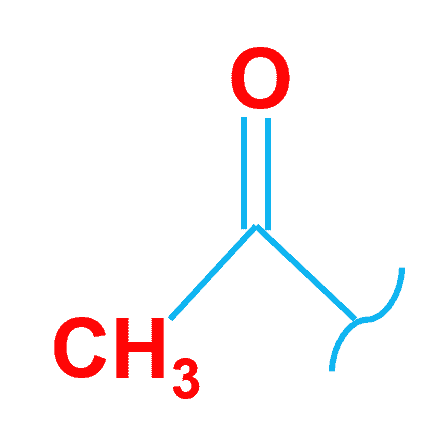
The hydrogen atom on the carboxylate group in methanoic acid HCOOH⇌H++HCOO− is the acidic hydrogen (the one bound directly to the carbon atom does not dissociate).
Table of Contents
More Interesting Topics
Light Energy| 5- Easy Examples
Perchloric Acid| Is It Super Acid?
Sulfurous Acid Formula & Lewis Structure
CH4 Lewis Structure & Molecular Geometry
Electric Field Units & Definition
Mass Vs Weight| 5 Easy Examples
Latest posts by Umair Javaid, PhD Student (see all)
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023