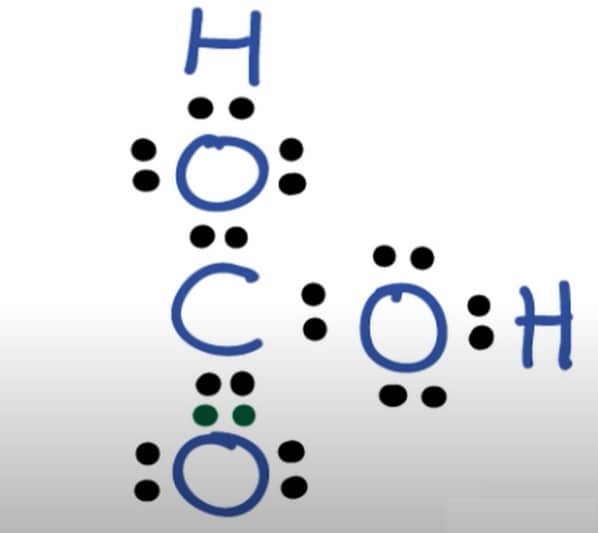
Carbonic acid (H2CO3) is a weak inorganic acid that only exists as a solution. Aerial acid, carbonic acid, and dihydrogen carbonate are all names for carbonic acid. It forms two kinds of salts: carbonates and bicarbonates. It can also be found in rainwater, calcite, fermentation, coal, groundwater, meteors, volcanoes, amino acids, proteins, oceans, plants, sulfur deposits, salts, and caves.
https://www.youtube.com/watch?v=spcvvWKudFQ
| Name of molecule | Carbonic acid (Grayish white solid) |
| Other names | Aerial acid, carbonic acid, and dihydrogen carbonate |
| Boiling point | -78 degree Celsius |
| Melting point | 210 Celsius |
| The molar mass of HClO4 | 100.454 g/mol |
| Molecular weight | 62.024 g/mol |
| Other names | diprotic acid, which means that it has two protons that can disassociate from the parent molecule |
| The ph of carbonic acid | 4.68 in 1mM |
| Uses of Carbonic acid | Widely used in making bubbly, fizzy drinks cleaning contact lenses hydrolysis of starch |
Table of Contents
Carbonic Acid Preparation
Carbonic acid can be made by reacting strong acids such as hydrogen chloride with calcium carbonate and allowing the resulting carbon dioxide to pass into the water.
CaCO3 + HCl → CaCl2 + CO2 ↑+ H2O
CO2 + H2O → H2CO3 (Carbonic acid)
Molar Mass of Carbonic Acid
Oxygen molar mass =15.9994 g/mol.
Hydrogen molar mass = 1.00794 g/mol.
Molar mass of Carbon= 12.01g/mol
Molar mass of H2CO3 = 2(1.00794)+12.01+3(15.9994)
=62.03 g/mol
Carbonic Acid Uses
- Carbonic acid is widely used in the production of bubbly beverages such as sodas, soft drinks, sparkling wines, and other aerated beverages.
- H2CO3 is used in the precipitation of many ammonium salts such as ammonium persulfate.
- Carbonic acid solutions are extremely effective at cleaning contact lenses.
Is H2CO3 a strong acid?
H2CO3 is a weak acid that reacts to form a proton (H+ cation) and a bicarbonate ion (HCO3- anion). In aqueous solutions, this compound only partially dissociates.
A strong acid ionizes completely or almost completely in water.

Important Links
Related Links
N2O Lewis Structure| Laughing Gas
SO2 (Sulfur Dioxide) Lewis structure
NH3 Lewis Structure & Molecular Geometry
CH4 Lewis Structure & Molecular Geometry
Frequently Asked Questions (FAQs)
1. Is sodium sulfate the same as salt?
Sodium sulfate is an inorganic chemical with the formula Na2SO4.
It is primarily used in the manufacture of detergents and the Kraft process of paper pulping. It can be found in all forms as white solids that are highly water-soluble. Decahydrate is a significant commodity chemical product, with an annual output of 6 million tonnes.
2. What is sulfur electronic configuration?
Sulfur electronic configuration is 1s2 2s2 2p6 3s2 3p4
3. What is CLF3 molecular geometry?
ClF3 has a T-shaped molecular geometry and trigonal bipyramidal electron geometry. This molecule has two lone pairs and three bound pairs, according to the ClF3 Lewis structure. ClF3 is a polar compound.
4. Why is flaring necessary?
Flaring is a method of disposing of flammable materials, mostly methane, as well as reducing odor irritants, health concerns, and negative environmental effects.
5. What is a volatile substance?
The term “volatile” refers to compounds with a high evaporation capability. They have fewer intermolecular interactions and, as a result, may be transferred to the vapor phase quickly. They also have higher vapor pressures and lower boiling temperatures.
6. What are ionic compounds?
Ionic compounds are formed by a process known as electron transfer, in which one atom transfers electrons to another. An atom of one element loses one or more electrons during electron transfer, and an atom of another element gains those electrons. Both atoms are involved in the electron transfer from ions.
7. What is convection in the atmosphere?
Convection in the atmosphere is frequently seen in our weather system. As the sun heats the Earth’s surface, the air above it warms and rises. If the conditions are favorable, this air can continue to rise, cooling and producing Cumulus clouds.
8. What is the process of distillation?
Distillation is the separation of a mixture of liquids based on variations in their boiling points (or volatility). Water may be extracted from a salt solution using this method.
9. What is the combustion process?
Combustion is a chemical process in which heat and light are produced. The most common sort of combustion is fire.
10. Is zinc a strong metal?
Zinc metal is a low-tensile-strength metal with less than half the tensile strength of mild carbon steel.
SO3 (sulfur trioxide) is a chemical compound. It comes in three different forms: gaseous monomer, crystalline trimer, and solid polymer. It is solid at temperatures slightly below room temperature and has a relatively narrow liquid range. The primary precursor to acid rain is gaseous SO3.
Carbonic acid is not considered toxic or hazardous to human health because it occurs naturally in the human body. It should be noted, however, that high concentrations of H2CO3 can irritate the respiratory tract and the eyes.
More Interesting Links
Disclaimer
Whatsinsight.org‘s blog and everything published on it are provided solely as an information resource. The blog, its authors, and affiliates accept no responsibility for any accident, injury, or damage caused by following the information on this website, in part or in whole. We do not recommend using any chemical without first consulting the manufacturer’s Material Safety Data Sheet and following the safety advice and precautions on the product label.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023