Sodium Sulfate is the sodium salt of sulfuric acid. It’s an inorganic compound with the formula Na2SO4.
It is mainly used for the manufacture of detergents and in the Kraft process of paper pulping. All of its forms are white solids that are extremely water-soluble. Decahydrate is an important commodity chemical product, with an annual production of 6 million tonnes.
Keep reading to know more about the Lewis structure and production of Na2SO4 along with details about Glauber’s Salt.

Table of Contents
Sodium Sulfate Lewis Structure
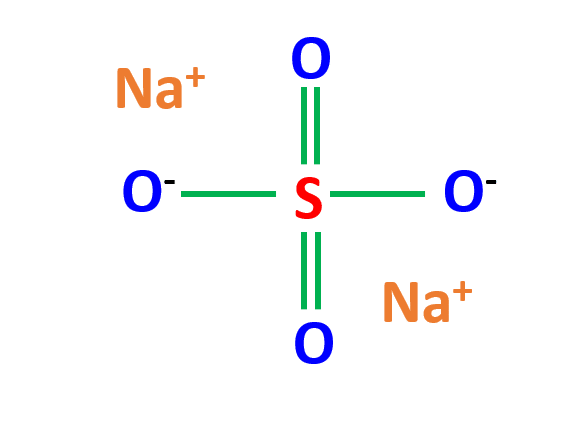
From the Na2SO4 Lewis structure, it can be noticed that two Sodium (metal) atoms are bonded to a group of non-metal atoms (sulfur and oxygen).
Sodium has one valence electron. It transfers that valence electron to the sulfate ion to form an ionic bond with sulfate ions.

Key Points
- White crystalline solid (Anhydrous sulfate).
- Inorganic compound with formula Na2SO4.
- Generally regarded as non-toxic.
- Odorless and soluble in water.
- Insoluble in ethanol.
- Its crystallized product, (Na2SO4·10H2O), is commonly known as Glauber’s salt.
- Not expensive material. Its largest use is as filler in powdered home laundry detergents.
- Used as a diluent for food colors.
- Boiling point is 1,429 °C (anhydrous).
- Density is 2.664gm/ml (anhydrous), 1.464gm/ml (decahydrate).
- It is 32.37% sodium and 67.63% sulfate.
- Very efficient at removing dirt.
What is Glauber’s Salt?
Glauber salt (Na2SO4.10H2O) is sodium sulfate salt-containing ten water of crystallization. Its other name is mirabilite.
Mirabilite is the natural mineral form of the decahydrate. About two-thirds of the world’s production of Na2SO4 is obtained from mirabilite. It is also produced as a by-product in hydrochloric acid production.
It is known to be effective for the removal of certain drugs such as paracetamol from the body in the case of overdose.
Molar Mass of Anhydrous Na2SO4
Molar mass of Na = 22.99 g/mol.
Sulfur Molar mass = 32.07 g/mol.
The molar mass of Oxygen = 16.00 g/mol
Molar mass of Na2SO4 = 2x Atomic mass of Na + Atomic mass of S + 4x Atomic mass of O.
=2x(22.99)+32.07+(4×16.00).
Molar mass=142.04 g/mol.
Mass % of different elements present in Na2SO4
Mass % of Sodium = (2x Atomic mass of Na x 100)/(molar mass of Na2SO4)
=2×23/142=32.39%
Mass % of Sulphur = (Atomic mass of S x 100)/(molar mass of Na2SO4)
=32×100/142 =22.53%
Mass % of Oxygen = (4x Atomic mass of O x 100)/(molar mass of Na2SO4)
=4x16x100/142 = 45.07 %
How can Na2SO4 be Prepared?
It can be prepared by reacting sulfuric acid with common salt.
H2SO4 + 2NaCl → Na2SO4 + 2HCl
It can also be prepared via the Hargreaves process, as shown below:
4NaCl + 2H2O + 2SO2 + O2 → 2Na2SO4 + 4HCl
Uses Of Na2SO4
- In the laboratory, anhydrous Na2SO4 is widely used as an inert drying agent, for removing traces of water from organic solutions.
- Na2SO4 is used in the Kraft process for the manufacture of wood pulp.
- It is a common component of seawater and many saline or alkaline lakes.
- Used to remove small air bubbles from molten glass.
pH of Na2SO4
Na2SO4 is a neutral salt (pH of 7). The neutrality is due to fact that Na2SO4 is derived from a strong acid (sulfuric acid) and a strong base (sodium hydroxide).
Related Links
N2O Lewis Structure| Laughing Gas
HCN Lewis Structure| Step By Step Construction
SiO2 Lewis Structure| Step By Step Construction
Sublimation Definition| Explanation
SO2 (Sulfur Dioxide) Lewis structure
Safety data sheet of Na2So4
Sulfurous Acid| Formula & Lewis Structure
Frequently Asked Questions (FAQs)
1. What is sodium sulfate used for?
Na2SO4 is used in the production of detergents, rayon, wood pulp, the manufacturing of dyes and textile finishes. It is also used in the manufacturing of glass, paper, and shampoo.
It is used in soda to produce carbonation of soda.
2. Why is Na2SO4 a drying agent?
Na2SO4 is used as a drying agent, larger clumps of Na2SO4 absorb water from the solution.
After leaving for a short period, the crystals are removed by filtration, and the solution is then relatively free of water.
3. Is Na2SO4 a base?
Na2SO4 is neutral as it is a salt of a strong acid and a strong base.
It can be prepared by reacting sodium carbonate with dilute sulfuric acid.
4. Can Na2SO4 conduct electricity?
Na2SO4 is a strong electrolyte.
Electrolytes are particles that carry a positive or negative electric charge.
Its 0.10M aqueous solution is a better conductor of electricity than the 0.1M solution of NaCl.
4. What are sodium phosphate formula and molar mass?
Sodium phosphate formula is Na3PO4
molar mass = (3 x mol mass of Na) + mol mass of P+ (4 x mol mass of Oxygen)
molar mass of Na3PO4 =3×23+1×31+4×16=164gmol−1
Sodium Lauryl Sulfate is used as a surfactant in detergents. It lowers the surface tension between ingredients, which is why it’s used as a cleansing and foaming agent.
It’s a part of ingredients in beauty and self-care products as well as in household cleaners.
Sodium Sulfate is a neutral salt, which forms aqueous solutions with a pH of 7. It is obtained by strong acid (sulfuric acid) and a strong base (sodium hydroxide).
Author
Umair Javed
Umair has been working at Whatsinsight since 2020 as a content writer.
He has a Masters degree in Material Science.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023