Surface tension is a property of a liquid’s surface that allows it to resist external forces due to the cohesive nature of water molecules. The molecules at the surface have similar molecules on all sides, so they cohere more strongly with those who are directly associated with them on the surface. This results in the formation of a surface “film,” which makes moving an object through the surface more difficult than moving it when completely submerged.
Table of Contents
Surface Tension of Water-Simple Words
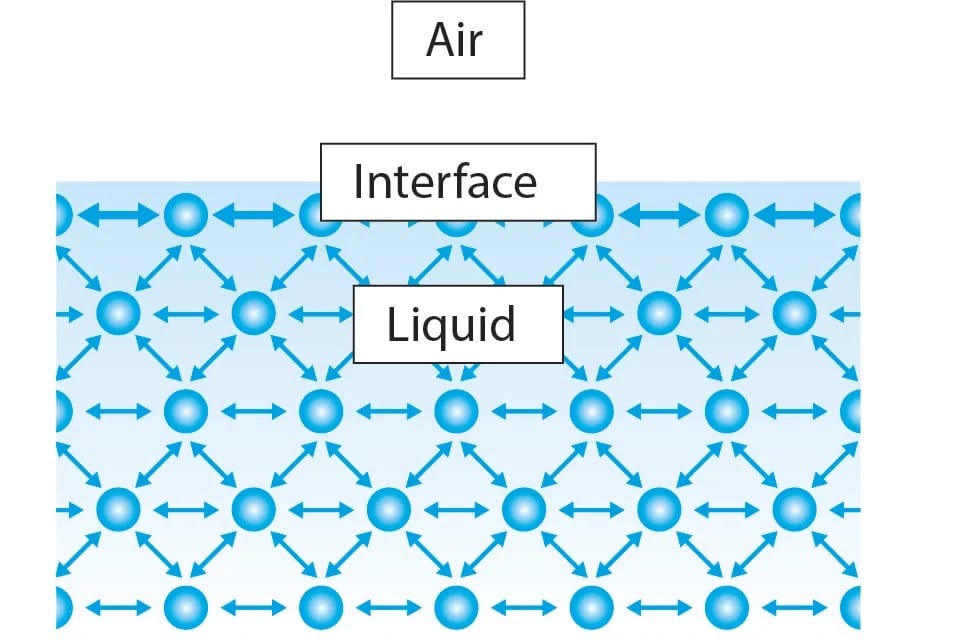
A water sample contains two types of molecules. Those on the outside are known as the “exterior,” while those on the inside are known as the “interior.” Interior molecules are attracted to all molecules in their vicinity, whereas exterior molecules are only attracted to those on the surface and below the surface.
At the interface, the water molecules have only half of the neighboring water molecules compared to the bulk of the liquid.
As a result, the energy state of molecules on the inside is significantly lower than that of molecules on the outside. As a result, the exterior molecules try to keep a small surface area, allowing more molecules to be in a lower energy state. This is what causes water’s surface tension.
In the water molecule, two hydrogen atoms form a covalent bond with an oxygen atom. Because of its high electronegativity, oxygen will have a large portion of the negative charge on its side, whereas hydrogen will be more positively charged. As a result, the hydrogen atoms of one molecule attract the oxygen atoms of another. They formed bonds are known as hydrogen bonds, and they result in strong cohesive forces between water molecules as well as a high water surface tension.
The surface tension of water is 0.07275 joule per square meter at 20 °C (68 °F).
Surface tension is measured by the amount of force (N) exerted on a unit such as a length (m) or the amount of energy of a measured area.
The surface tension equation is given by the equation S = (ρhga/2) where S is the surface tension, (or rho) is the density of the liquid being measured, h is the height of the liquid in the tube, g is the acceleration due to gravity acting on the liquid (9.8 m/s2), and a is the radius of the capillary tube.
Surface Tension Formula
The surface tension of water is a physical property that represents the energy required to increase the surface area of the water by a unit amount. The formula to calculate the surface tension of water is:
𝛾 = F ÷ L
where 𝛾 is the surface tension of water in units of energy per unit area (such as N/m or dyn/cm), F is the force required to increase the surface area of the water by a unit amount, and L is the length of the line over which the force is acting.
For water at room temperature and standard pressure, the surface tension is about 72.8 millinewtons per meter (mN/m) or 72.8 dynes per centimeter (dyn/cm).
Units of Surface Tension
- Newton per meter
- Dyne per centimeter
- Joules per meter squared
Daily Life Examples of Surface Tension
- Soaps and detergents help clean garments by lowering water surface tension, allowing water to more easily penetrate pores and dirty areas.
- Hot water is preferred for washing because it has lower surface tension and is a better wetting agent.
- The surface tension of water provides the necessary wall tension for the formation of bubbles. The tendency to reduce wall tension causes the bubbles to round.
- Surface tension shapes liquid droplets. The surface layer’s cohesive forces tend to draw water droplets into a spherical shape.
- Water striders and other small insects can walk on water because their weight does not penetrate the surface.
- Despite being several times denser than water, a small needle can float on the surface of the water.
- Water surface tension bridges the pores of finely woven tent materials, making them rainproof.
- Disinfectants are typically low surface tension solutions. This allows them to spread and damage bacterial cell walls.
- When water forms droplets, the surface tension of the water molecules holds the droplet together, giving it a round shape.
- When a liquid is poured onto a surface, the surface tension of the liquid causes it to spread out and wet the surface. For example, when water is poured onto a glass surface, it spreads out to cover the entire surface.
- When a narrow tube is placed into a liquid, the surface tension of the liquid causes it to rise up the tube, a phenomenon called capillary action. This is why water can climb up a thin straw or why ink is drawn up into a fountain pen.
Summary
- Surface tension is a property of a liquid’s surface that allows it to resist external forces due to the cohesive nature of water molecules.
- The units of surface tension are Newton per meter, Dyne per centimeter, and Joules per meter squared.
- The high surface tension of water is caused by strong molecular interactions.
- All liquids have the surface tension property.
- In a water molecule, the electronegativity difference between oxygen and a hydrogen atom is a responsible factor for water’s surface tension.
Frequently Asked Questions
1. Why surface tension of mercury is greater than water?
Mercury has very high surface tension. Because mercury is a metal, the molecules’ bonds are metal bonds, which are much stronger than hydrogen bonds in a water molecule, resulting in extremely high cohesive forces and surface tension.
2. Is water vapor a greenhouse gas?
Yes, water vapors are greenhouse gas. other examples are listed below:
- Carbon dioxide
- Methane
- Ozone
- Nitrous oxide
- Chlorofluorocarbons
3. What is the weight of water?
1 liter of water should weigh 1 kilogram.
1 Liter Equals 1000 cm3 and 1 kilogram = 1 Liter of water
At 17 °C, one US gallon of water weighs 8.345 pounds, or 3.785 kg, but one Imperial gallon of water weighs 10.02 pounds, or 4.545 kg. Click here to find more details about the weight of water.
4. What is the density of water?
At 4.0°C (39.2°F), the density of water in g/ml is 0.9998395. This is equivalent to one gram per milliliter (g/ml) or one gram per cubic centimeter (g/cm3). Check full article density of water g/ml.
5. How many cups are in a gallon?
A US liquid gallon is equal to 16 cups, while a US dry gallon is equal to 18.61 cups. In the United States, one cup equals half a pint (236.6 ml). Check the full article “How many cups are in a gallon”.
More Interesting Topics
Neon Element
Liquid Oxygen-Cryogenic Liquid
How cold is Liquid Nitrogen?
Electron Configuration for Calcium
Is Air a Homogeneous Mixture?
The pH of Distilled/ De-Ionized Water
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023



