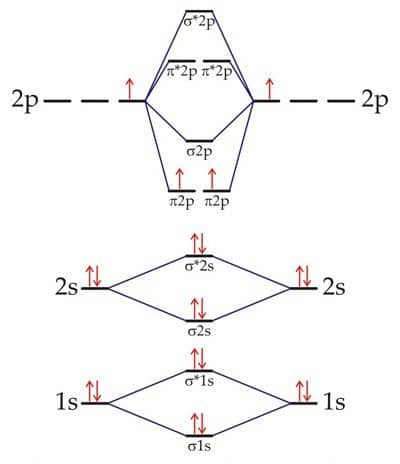
The atomic orbitals of both boron atoms overlap to produce the B2 molecule. Each boron atom contains three valence electrons. Three valence electrons from each boron atom, for a total of six, must be accommodated in various molecular orbitals in ascending order of their energies to form the B2 molecule.
B2 is paramagnetic in nature with two electrons; to form that bond, there are two half-pi bonds, which form what we represent improperly in inline notation as a bond, although it isn’t actually a sigma bond.
In paramagnetic materials, constituent atoms or molecules have permanent magnetic moments (dipoles), even in the absence of an applied field. The spin of unpaired electrons in atomic or molecular electron orbitals is generally responsible for the permanent moment.
Table of Contents
How B2 Is Paramagnetic in Nature?
In order to form a B2 molecule, three valence electrons from each boron atom, for a total of six, must be accommodated in various molecular orbitals in increasing order of their energies.
MO electronic configuration of B2 is (σ2s)2(σ∗2s)2(π2px,π2py)2
Bond order = 0.5 [Number of bonding electrons- Number of antibonding electrons ]
= 0.5[4-2] = 1
The two boron atoms in B2 are linked by one covalent bond.
Since each π2px and π2py contain unpaired electrons, therefore B2 molecule is paramagnetic.
Frequently Asked Questions (FAQs)
1. What is the acetyl group definition?
The acetyl group consists of a carbonyl group made up of a carbon double bonded to oxygen and a methyl group, which is simply a carbon with three hydrogens (CH3). It is a part of a larger family of the acyl group
2. Is an acetyl group a ketone?
Both groups contain carbonyl groups. However, a ketone has to have carbon on both sides of the carbonyl group. An acetyl group just needs a methyl group on one side and a carbonyl group on another side.
3. What is the acetyl group?
The acetyl group consists of two parts. The first part is a carbonyl group made up of a carbon double bonded to oxygen. A second part is a methyl group, which is simply a carbon with three hydrogens (CH3).
4. What is the molar mass of acetic acid?
The molar mass of acetic acid is 60.052 g/mol. The acetic acid has the formula CH3COOH and it is also known as Ethanoic acid. The pH of a 1M solution of CH3COOH is 2.4.
5. Is SiCl4 polar or nonpolar?
SiCl4 (silicon tetrachloride) is a nonpolar molecule. Because the four chemical bonds between silicon and chlorine are uniformly distributed, SiCl4 is non-polar. A polar covalent bond is a type of covalent link that is intermediate between pure covalent bonds and ionic bonds. When the difference in electronegativity between the anion and the cation is between 0.4 and 1.7, such bonds occur.
Check the full article “Is SiCl4 polar or nonpolar?”.
6. specific heat of air?
The specific heat of air at constant pressure is 1.005 kJ/kg K and the specific heat of air at constant volume is 0.718 kJ/kg K.
The specific heat (C), also called heat capacity, of a substance is the amount of heat required to raise its temperature by one degree.
7. hydrogen ion?
A hydrogen ion is the nucleus of a hydrogen atom that has been detached from its electron. The hydrogen nucleus is made up of a proton, which is a particle with a unit positive electric charge. As a result, the solitary hydrogen ion, indicated by the symbol H+, is widely employed to represent a proton.
8. Sublimation?
Sublimation is the process by which a solid turns straight into a gaseous state without first going through the liquid stage. Dry ice is one of several sublimation instances (frozen carbon dioxide). When dry ice is exposed to air, it immediately changes its phase from solid to gaseous (fog).
Vapor deposition is the inverse of sublimation.
9. Distillation?
Distillation is the separation of a mixture of liquids based on variations in their boiling points (or volatility). Water may be extracted from a salt solution using this method.
10. nitric acid?
Nitric acid (HNO3) is a colorless, fuming, and very corrosive liquid that is employed as a laboratory reagent as well as an important industrial chemical in the manufacture of fertilizers and explosives. It is toxic and can result in severe burns.
More Interested Links
H2S Lewis Structure & Molecular Geometry
CO Lewis Structure & Molecular Geometry
O2 Lewis Structure & Molecular Geometry
CO2 Lewis Structure and Molecular Geometry
SiO2 Lewis Structure
SO2 (Sulfur Dioxide) Lewis structure
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023



