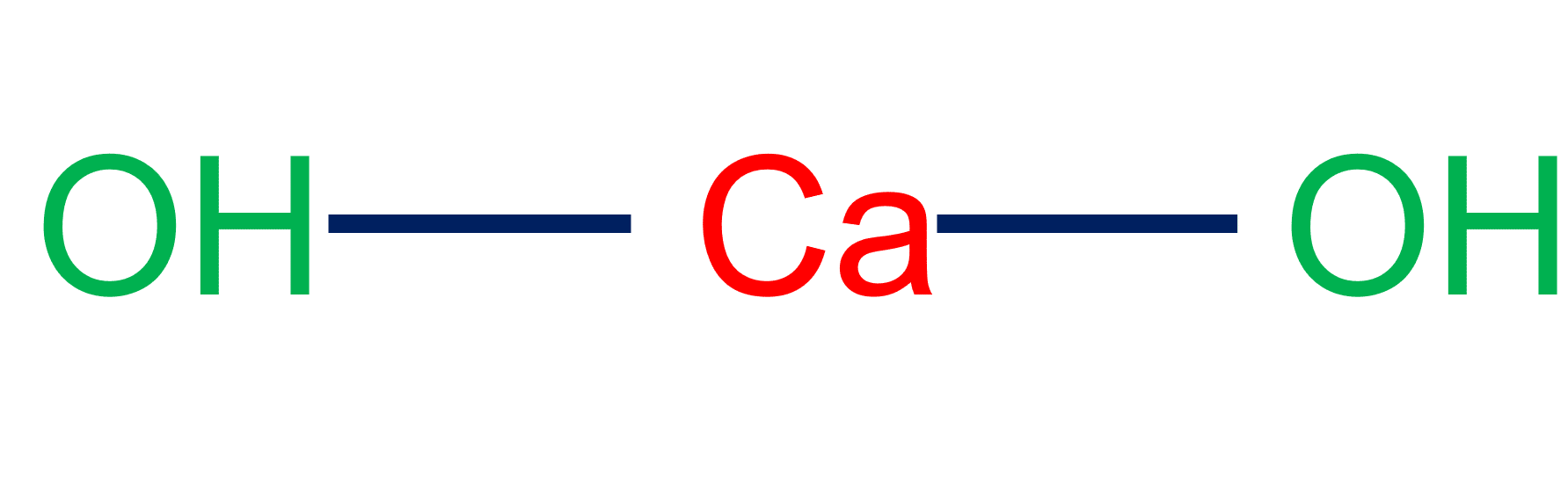Calcium oxide, CaO, is a white or greyish white solid produced in large quantities by roasting calcium carbonate to drive off carbon dioxide. The term “lime” refers to calcium-containing inorganic materials that are primarily composed of calcium carbonates, oxides, and hydroxides, as well as silicon, magnesium, aluminum, and iron. Quicklime, on the other hand, refers to the chemical compound calcium oxide as a whole. Free lime is calcium oxide that does not react in building products such as cement during processing. Quicklime is a relatively low-cost mineral. It, as well as one of its chemical derivatives (calcium hydroxide, of which quicklime is the base anhydride), are important commodity chemicals.
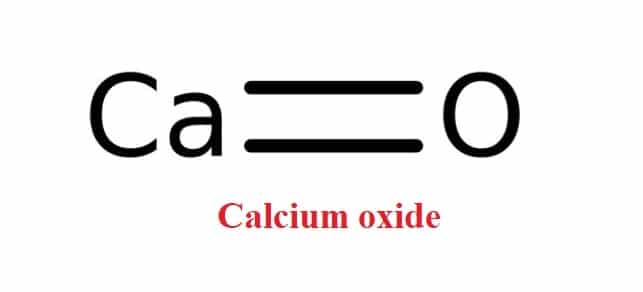
The calcium cation Ca+2 and the oxygen anion O-2 form an ionic bond to form the calcium oxide molecule. The cubic calcium oxide ionic lattice is similar to the NaCl lattice, with an ion surrounded by 6 opposite charge-ion.
| Technical Name of compound | Calcium oxide (CaO) |
| Other names | Lime and Quick Lime |
| Appearance | crystalline, alkaline, caustic, and white solid (at room temperature) |
| Melting point | 2,572° C (4,662° F) |
| Boiling point | 2850 °C (5162 °F) |
| Density | 3.2-3.4 g/mL |
| Uses | Used as an alkali for treating acidic soils and in manufacturing steel, paper, and glass, and it is the main component of lime |
Calcium oxide can be produced in a lime kiln by thermal decomposition of materials containing calcium carbonates (CaCO3; mineral calcite), such as limestone or seashells. Calcination is the process by which burnt lime is produced.
CaCO3 → CaO + CO2
The temperature range for calcination of calcium carbonate is 1070-127 oC. Typically, these reactions occur in a rotary kiln, and the products and results of those reactions are carbon dioxide and burnt lime. Following Le-principle, Chatelier’s the formed carbon dioxide is removed instantly, allowing the reaction to be preceded until the process is completed.
Table of Contents
Calcium Oxide-Key Points
- It releases heat when dissolved in water and changes into calcium hydroxide.
- Calcium oxide is also a base. It reacts with acids to make calcium salts.
- Quicklime is an important ingredient of cement.
- Calcination is the process by which burnt lime is prepared. At high temperatures, the reactants are thermally decomposed while the temperature remains below the melting point.
- Lime is used as a reagent in laboratories for precipitation reactions, dehydration, and other processes.
- When heated to 4,350 degrees Fahrenheit or 2,400 degrees Celsius, quicklime emits an intense glow. This type of illumination is known as the limelight, and it was widely used in theatrical productions prior to the invention of electric lighting.
Element Calcium
Calcium (Ca) is the fifth element and the third most abundant metal in the earth’s crust, with an atomic number of 20. It’s a trimorphic metal that’s harder than sodium but softer than aluminum. It has the same properties as beryllium and aluminum, but unlike alkaline metals, it does not cause skin burns. It has a lower chemical reactivity than alkaline metals and alkaline-earth metals.
Burnt Lime
Burnt lime is a highly reactive calcium oxide that can be used in road construction, steel production, paper production, and sludge stabilization.
Burnt lime is composed of calcium oxide with trace amounts of calcium carbonate and silica.
It is produced by heating calcium carbonate (limestone) to high temperatures (greater than 1200° C) in a kiln system.
Caustic Soda
Caustic Soda, also known as Sodium Hydroxide, has a wide range of applications, including drain cleaning and soap production. Caustic soda is commonly used as a drainpipe cleaner, to unclog drains, remove built-up grease from ovens, and make soap and detergents.
Summary
- Calcium oxide is a white solid compound with a very high melting point that reacts with water to form calcium hydroxide (Ca(OH)2). Commercially, it is frequently prepared by heating limestone.
- Calcium oxide is the main component of lime and is used as an alkali in the treatment of acidic soils as well as the manufacture of steel, paper, and glass.
- Free lime is calcium oxide that does not react in building products such as cement during processing.
- Quicklime is a relatively low-cost mineral. It, as well as one of its chemical derivatives (calcium hydroxide, of which quicklime is the base anhydride), are important commodity chemicals.
- Calcination is the process by which burnt lime is prepared.
Related Links
Molar Mass of Acetic Acid| Easy-Explanation
Acetyl Group| 9 Key Points-Easy Explanation
Hydrogen Bond| Definition & Easy Explanation
Is HCN Polar or Nonpolar| Simple Explanation
Liquid Oxygen-Cryogenic Liquid
Frequently Asked Questions
1. Why is flaring necessary?
Flaring is a method of disposing of flammable materials, mostly methane, as well as reducing odor irritants, health concerns, and negative environmental effects.
2. What is a volatile substance?
The term “volatile” refers to compounds with a high evaporation capability. They have fewer intermolecular interactions and, as a result, may be transferred to the vapor phase quickly. They also have higher vapor pressures and lower boiling temperatures.
3. What are ionic compounds?
Ionic compounds are formed by a process known as electron transfer, in which one atom transfers electrons to another. An atom of one element loses one or more electrons during electron transfer, and an atom of another element gains those electrons. Both atoms are involved in the electron transfer from ions.
4. What is convection in the atmosphere?
Convection in the atmosphere is frequently seen in our weather system. As the sun heats the Earth’s surface, the air above it warms and rises. If the conditions are favorable, this air can continue to rise, cooling and producing Cumulus clouds.
5. What is the process of distillation?
Distillation is the separation of a mixture of liquids based on variations in their boiling points (or volatility). Water may be extracted from a salt solution using this method.
6. What is the combustion process?
Combustion is a chemical process in which heat and light are produced. The most common sort of combustion is fire.
7. Is zinc a strong metal?
Zinc metal is a low-tensile-strength metal with less than half the tensile strength of mild carbon steel.
8. What are quarks?
In physics, quarks are the fundamental building units of matter. They’re most typically found within protons and neutrons, the fundamental components of the universe’s atoms. Based on present experimental findings, quarks appear to be really basic particles that cannot be further subdivided.
9. What is the difference between weathering and erosion?
Weathering and erosion are geological processes that contribute to the shaping of the Earth’s surface.
Weathering is the degradation of rocks, soils, and minerals due to direct contact with the Earth’s atmosphere. Erosion, on the other hand, is the movement of solids (soil, mud, rock, and other particles) downward or down-slope produced by current agents such as wind, water, or ice responding to gravity or by living organisms.
Please check the full article “Weathering vs erosion”.
More Links
Disclaimer
Whatsinsight.org‘s blog and everything published on it are provided solely as an information resource. The blog, its authors, and affiliates accept no responsibility for any accident, injury, or damage caused by following the information on this website, in part or in whole. We do not recommend using any chemical without first consulting the manufacturer’s Material Safety Data Sheet and following the safety advice and precautions on the product label.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023