A Daniell cell is an electrochemical cell that converts chemical energy into electrical energy. In order to generate electricity, the cell undergoes a variety of chemical reactions. The cell is made up of zinc and copper electrodes, with zinc acting as the anode and copper acting as the cathode. Both metals have been dipped in their respective salt solutions.
The Daniell cell is an advanced form of the Voltaic cell that uses two electrodes made of Zinc and Copper to produce a difference potential of 1.1 V. The circuit is charged by the cell in which electrons circulate after being produced at the anode and transferred to the cathode.
Working Principle of Daniell Cell
An electrochemical cell is a device that produces electric energy as a result of a spontaneous redox reaction. This type of cell is also known as a Galvanic cell or a Voltaic cell, after two Italian scientists, Luigi Galvani (1780) and Alessandro Volta (1800), who were the first to experiment with chemical reactions and electric current. A common example of a Galvanic cell is the Daniel Cell.
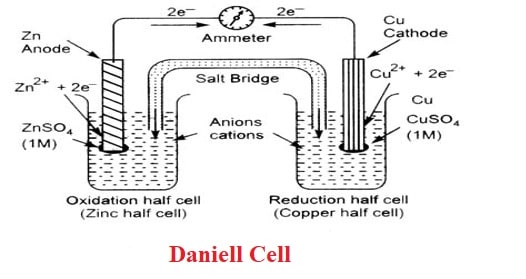
In the Daniell Cell, a copper vessel containing saturated Copper Sulfate (CuSO4) and diluted Hydrogen Sulfate is used (H2SO4). The Copper Sulfate solution acts as a depolarizer, and the Hydrogen Sulfate solution acts as an electrolyte. A consolidated Zinc rod is immersed in a Zinc Sulfate solution (ZnSO4).
A transparent layer can be seen just below the uppermost surface of the copper vessel, where the Copper Sulfate crystals and solution remain in contact. As a result, the solution can stay saturated indefinitely.
The working steps of Daniell’s cell are listed below:
- Electric current flows when two electrodes (zinc and copper rods) are connected externally by a wire (as shown by the ammeter).
- Zinc ions are formed when zinc ions dissolve from the zinc rod, whereas copper ions deposit on the topper rod as metallic copper.
- In the circuit, a voltmeter measures the potential difference between the two electrodes. This is the cell’s electromotive force (e.m.f).
- Oxidation occurs at the zinc electrode. So this is the anode: electrons released at this electrode travel through the wire as shown in the Figure to the copper electrode (the cathode), where they combine with copper ions to produce copper by reduction.
- As long as the electrodes are connected by a wire, electrons will be released at the anode and flow to the cathode (current will flow) until either the zinc rod or the copper ions are depleted.
- The solutions in both compartments remain electrically neutral.
- Zinc Sulfate concentration rises while Copper Sulfate concentration falls.
Equations for the two half-reactions are shown below:
Zinc electrode (anode): Zn(s) → Zn2+ (aq) + 2e– … … … (1)
Copper electrode (cathode): Cu2+ (aq) +2e– → Cu (s) … … … (2)
The sum of reactions (1) and (2) gives the complete cell reaction:
Zn (s) + Cu2+ (aq) → Zn2+ (aq) + Cu (s).
Daniell Cell in Simple Words
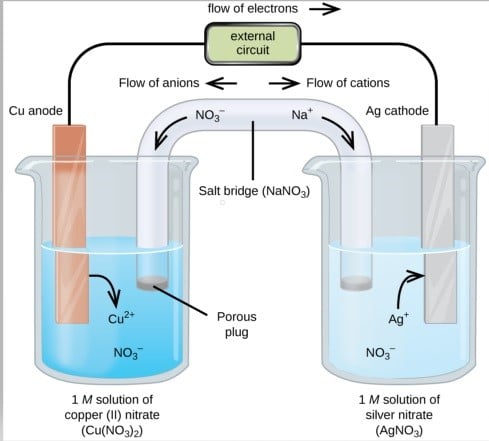
The Daniell cell is an electrochemical cell that produces a voltage difference between two metal electrodes immersed in an electrolyte solution. It was invented by the British chemist John Frederic Daniell in 1836 and was an important early device in the development of electrical technology.
The Daniell cell consists of a copper electrode and a zinc electrode that are both immersed in a solution of copper sulfate and zinc sulfate, respectively. The two electrolyte solutions are separated by a porous ceramic barrier or salt bridge that allows ions to pass between them but prevents the mixing of the two solutions.
The reaction that takes place in the Daniell cell is a redox reaction in which zinc atoms at the zinc electrode lose electrons and go into solution as zinc ions, while copper ions in the copper sulfate solution gain electrons and deposit as copper atoms on the copper electrode. This flow of electrons creates an electrical current that can be used to power electrical devices. The voltage produced by a Daniell cell is approximately 1.1 volts.
Real-Life Examples of Daniell Cell
The Daniell cell is not commonly used in daily life as it has been largely replaced by more efficient and practical power sources such as batteries and mains electricity. However, it played an important role in the development of electrical technology and is still used in some educational settings to demonstrate the principles of electrochemistry and circuitry.
Some possible examples of where the Daniell cell might be used in daily life include:
- Educational demonstrations: The Daniell cell is often used in educational settings to demonstrate the principles of electrochemistry and circuitry.
- Historical reenactments: The Daniell cell is sometimes used in historical reenactments to show how early electrical devices worked.
- Science experiments: The Daniell cell is still used in some science experiments to investigate the principles of electrochemistry, such as the effects of changing the concentration of the electrolyte solutions or the type of metal used for the electrodes.
- Art conservation: The Daniell cell has been used in art conservation to restore the colors of certain works of art, such as 19th-century paintings, by selectively reducing and removing metal ions from the pigments.
Summary
- A Daniell cell is a type of galvanic cell that converts chemical energy to electrical energy.
- The cell is made up of zinc and copper electrodes, with zinc acting as the anode and copper acting as the cathode.
- Electrons always flow from the anode to the cathode or from the oxidation half-cell to the reduction half-cell.
- The voltage of the Daniell Cell is 1.1 V.
Frequently Asked Questions
1. What is a salt bridge?
A salt bridge refers to a device used to form an electrochemical cell by providing a means to support the free flow of ions between the oxidation and reduction of half-cell components. A salt bridge facilitates corrosion because corrosive reactions typically occur in the presence of electrochemical cells.
2. What is a galvanic cell?
A galvanic cell is an electrochemical cell that generates an electric current through redox reactions.
This cell is powered by a spontaneous chemical reaction that generates an electric current that is then routed through an external circuit.
Energy is generated by galvanic cell reactions, which are then used to perform work.
3. What is a Daniell cell and how does it work?
- The Daniell cell is an electrochemical cell that produces a voltage difference between two metal electrodes immersed in an electrolyte solution.
- It consists of a copper electrode and a zinc electrode that are both immersed in a solution of copper sulfate and zinc sulfate, respectively.
- The two electrolyte solutions are separated by a porous ceramic barrier or salt bridge that allows ions to pass between them but prevents the mixing of the two solutions.
- The reaction that takes place in the Daniell cell is a redox reaction in which zinc atoms at the zinc electrode lose electrons and go into solution as zinc ions, while copper ions in the copper sulfate solution gain electrons and deposit as copper atoms on the copper electrode.
- This flow of electrons creates an electrical current that can be used to power electrical devices.
4. What is the voltage produced by a Daniell cell?
The voltage produced by a Daniell cell is approximately 1.1 volts.
5. What are some factors that can affect the voltage and performance of a Daniell cell?
Some factors that can affect the voltage and performance of a Daniell cell include the concentration of the electrolyte solutions, the type of metal used for the electrodes, the temperature of the cell, and the quality of the salt bridge or porous barrier separating the two solutions.
6. Paramagnetic materials?
Paramagnetic materials are metals that are weakly attracted to magnets. Aluminum, gold, and copper are among them.
7. What are some modern-day applications of the principles behind the Daniell cell?
- While the Daniell cell is not commonly used in daily life, the principles behind it are still important in many modern-day applications, such as in batteries, fuel cells, and other electrochemical devices.
- Understanding electrochemistry and circuitry is also essential for many areas of science and engineering, including materials science, chemical engineering, and electrical engineering.
More Interesting Topics
Combustion Reactions| Introduction, Reaction, & Facts
CH4 Lewis Structure & Molecular Geometry
Is Carbon Dioxide a Pure Substance?
Sigma Bond- Definition & Explanation
Distillation| Principles, and Processes
Delocalized pi bond
Physical Weathering| Short Overview
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023