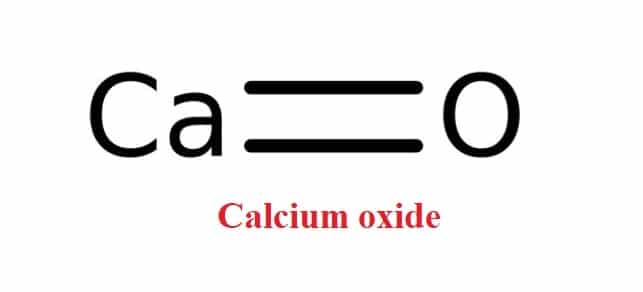
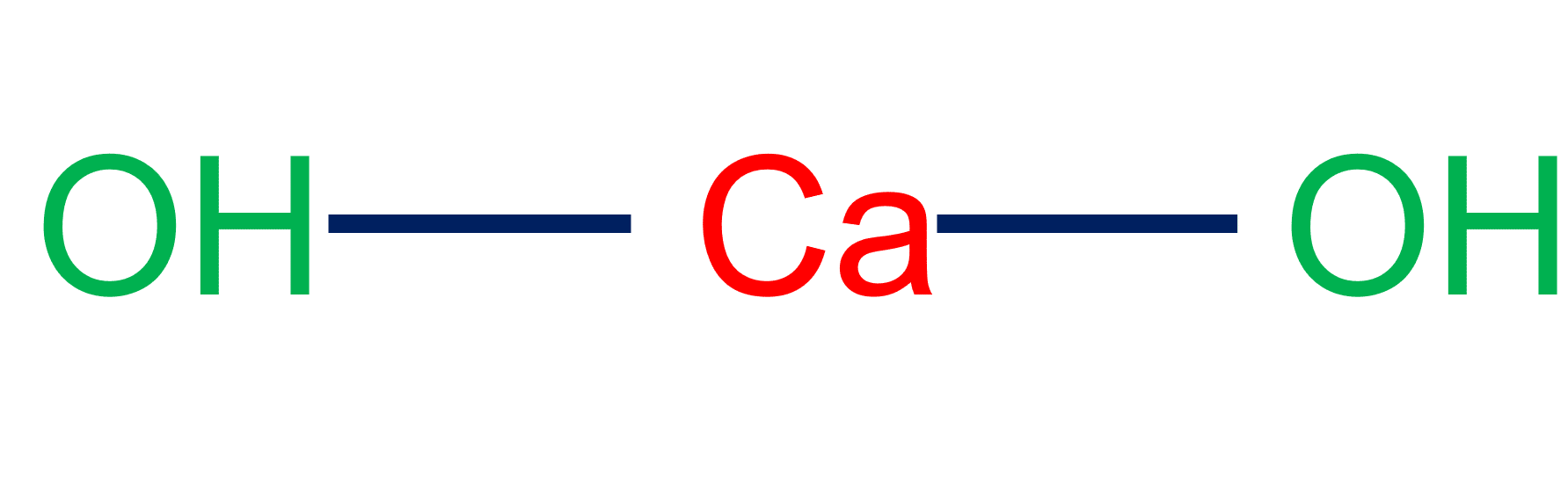Soda-lime (CaHNaO2) is a granular material made by saturating quicklime (CaO) with a strong solution of sodium hydroxide, NaOH (aq). Soda-lime functions in chemical reactions similar to sodium hydroxide, however unlike sodium hydroxide, it is not deliquescent and does not attack the glass.
| Technical Name of compound | Soda-lime (CaHNaO2) |
| Other names | hydrated lime, slack lime |
| Appearance | white or grayish-white color |
| Density | 2.5 g/cubic centimeter |
| Uses | Used in the Production of glass, ceramics, chemicals, and pharmaceuticals. Used as food additives. |
Soda-lime is a white or greyish white granular calcium hydroxide mixed with sodium hydroxide or potassium hydroxide. Soda-lime collects carbon dioxide and water vapor and degrades quickly unless stored in sealed containers. Soda-lime is used in medicine to absorb carbon dioxide in basal metabolism testing and rebreathing anesthetic systems. It is used as an absorbent for harmful gases in gas masks. It is used as a drying agent in labs. Soda-lime is a highly caustic toxin that, if consumed, causes serious damage to the gastrointestinal tract and may result in death.
Table of Contents
Soda-Lime Glass
The most common form of glass is soda-lime glass. Soda-lime-silica glass is another name for this. It is commonly used for windowpanes and glass containers such as bottles and jars for beverages, food, and certain commodities. Soda-lime glass accounts for over 90% of all produced glass.
Soda-lime glass is made up of around 70% silica (silicon dioxide), 15% soda (sodium oxide), and 9% lime (calcium oxide), with considerably lesser quantities of additional components.
Related Links
Is NH3 Acid or Base?| NH3 Properties
The pH of Distilled/ De-Ionized Water
Acidic Hydrogen
Sodium Phosphate – Formula, Structure, Types, and Uses
Density of Water in g/ml-Accurate Value
Frequently Asked Questions
1. What are ionic compounds?
Ionic compounds are formed by a process known as electron transfer, in which one atom transfers electrons to another. An atom of one element loses one or more electrons during electron transfer, and an atom of another element gains those electrons. Both atoms are involved in the electron transfer from ions.
2. What is the process of distillation?
Distillation is the separation of a mixture of liquids based on variations in their boiling points (or volatility). Water may be extracted from a salt solution using this method.
3. What is the combustion process?
Combustion is a chemical process in which heat and light are produced. The most common sort of combustion is fire.
4. Is zinc a strong metal?
Zinc metal is a low-tensile-strength metal with less than half the tensile strength of mild carbon steel.
5. What is Soda Lime Definition?
Soda-lime is a compound consisting of sodium hydroxide and calcium carbonate. It is utilized in a range of applications, including fire extinguishers and the production of glass and ceramics. It is also used to make soap and as a pH adjuster in swimming pools.
More Links
NH3 Lewis Structure & Molecular Geometry
How Much is a Liter of Water?| Glasses, Bottles, Cups, and
How Many Fluid Ounces Equal a Gallon
How Many Cups in a Gallon? Cups to Pints, Quarts, and More
How cold is Liquid Nitrogen?
Disclaimer
Whatsinsight.org‘s blog and everything published on it are provided solely as an information resource. The blog, its authors, and affiliates accept no responsibility for any accident, injury, or damage caused by following the information on this website, in part or in whole. We do not recommend using any chemical without first consulting the manufacturer’s Material Safety Data Sheet and following the safety advice and precautions on the product label.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023